Your Beer lambert law example images are available. Beer lambert law example are a topic that is being searched for and liked by netizens now. You can Find and Download the Beer lambert law example files here. Get all royalty-free images.
If you’re searching for beer lambert law example pictures information related to the beer lambert law example topic, you have visit the ideal blog. Our website always provides you with suggestions for downloading the highest quality video and image content, please kindly surf and find more enlightening video content and graphics that match your interests.
Beer Lambert Law Example. The important use of the Beer Lambert law is found in electromagnetic spectroscopy. Beer-Lambert law is the combination of 2 laws given by Johann Lambert and August Beer hence it came to be known as Beer-Lambert law. Beer-Lambert Law Equation The Beer-Lambert law equation is as follows. Its molar absorptivity is 8400 M -1 cm -1.
 How To Find The Path Length Using The Beer Lambert Law Chemistry Study Com From study.com
How To Find The Path Length Using The Beer Lambert Law Chemistry Study Com From study.com
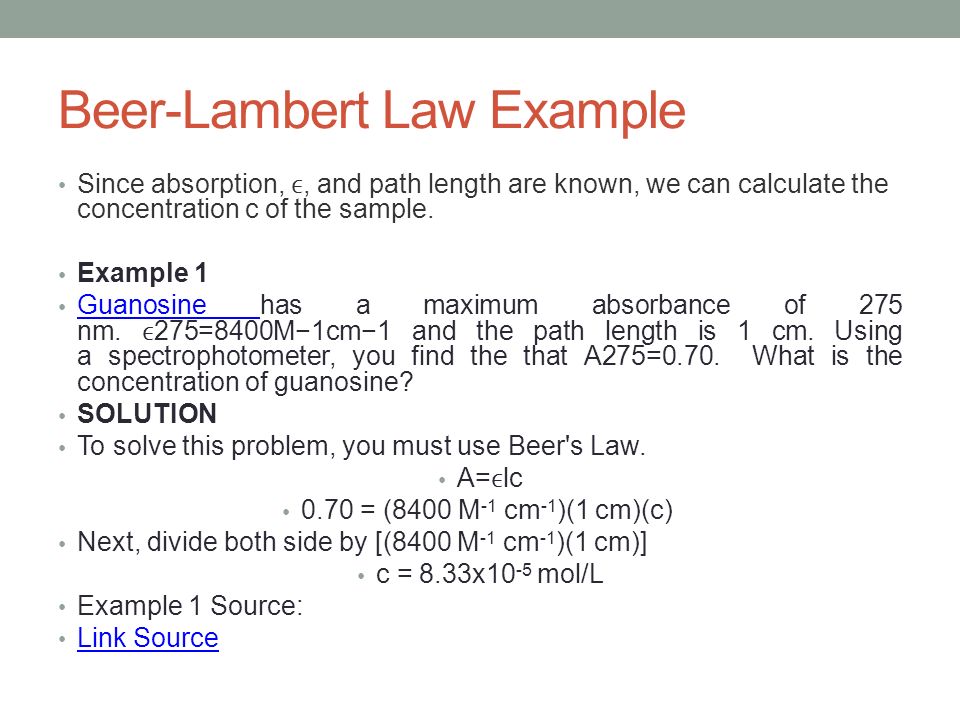
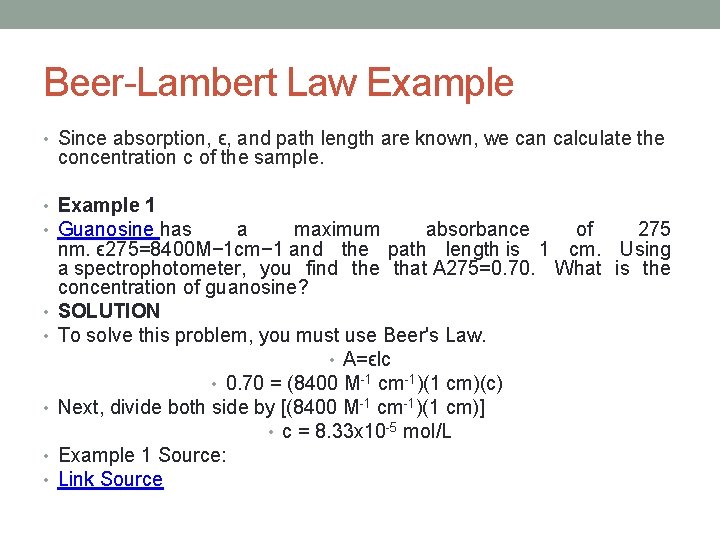
So c Aε l 054 64 10 3 05 Answer 0000168 M 2. The Beer-Lambert law data analysis. There is no information in this law about the nature of light. Its molar absorptivity is 8400 M -1 cm -1. To analyze the drugs for that lets take an example of a tablet. Where you able to determine the concentration of the.
The molar absorption coefficient is a sample dependent property and is a measure of how strong an absorber the sample is at a particular wavelength of light.
This implies that the increase of the concentration value gives an increasing. Where you able to determine the concentration of the. I I 0eμx I I 0 e μ x Where I is the intensity I0 is the initial intensity μ is the coefficient of absorption x is the depth in meter. According to the calibration curve the volume percent of the solution is 626. Though we may know the drug then the question. The general Beer-Lambert law is usually written as.
 Source: study.com
Source: study.com
Where you able to determine the concentration of the. Section 4 details how to fix this problem and a toy example of calibration is developed in Section 5 to show how the two calibration curves are. Lets suppose we have a tablet and we dont know which drug is present in it. A spectrophotometer finds A 070. What is the concentration of the sample.
 Source: slideplayer.com
Source: slideplayer.com
A spectrophotometer finds A 070. Beers Law Example Calculation. So c Aε l 054 64 10 3 05 Answer 0000168 M 2. Beer-Lambert Law and other calibration curves. Beers law in spectroscopy a relation concerning the absorption of.
 Source: aavos.eu
Source: aavos.eu
Calculating concentration using the BeerLambert law. To analyze the drugs for that lets take an example of a tablet. You have been provided with a Microsoft Excel spreadsheet containing two tabs. Beer Lambert Law Applications. A a λ b c.
 Source: slidetodoc.com
Source: slidetodoc.com
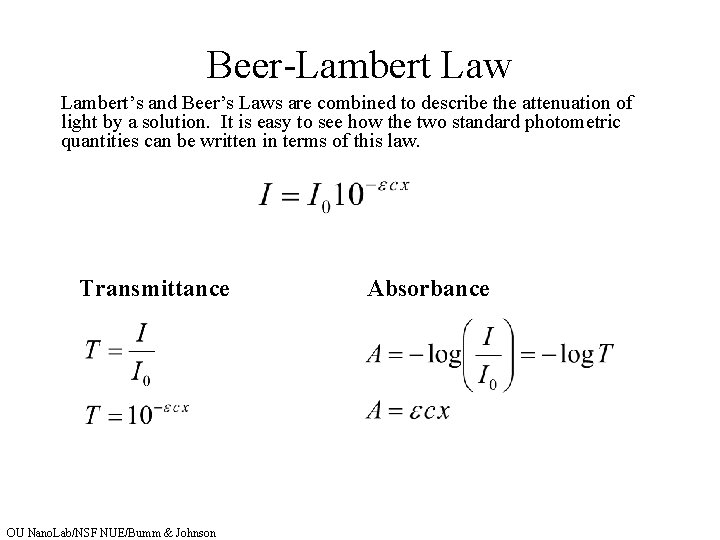
Beer-Lambert Law for example a cuvette filled Beers law. This implies that the increase of the concentration value gives an increasing. You have been provided with a Microsoft Excel spreadsheet containing two tabs. Lambert law states that absorbance and path length are directly proportional and it was stated by Johann Heinrich Lambert. There is no information in this law about the nature of light.
 Source: chegg.com
Source: chegg.com
Beer Lambert Law Applications. Beer-Lambert Law Equation The Beer-Lambert law equation is as follows. The Beer-Lambert Law implies that both the type and the concentration of the molecules are important in the process of radiation absorption. Where A is the measured absorbance a λ is a wavelength-dependent absorptivity. Lambert law states that absorbance and path length are directly proportional and it was stated by Johann Heinrich Lambert.
 Source: slidetodoc.com
Source: slidetodoc.com
The Beer-Lambert law data analysis. What is the concentration of the sample. A a λ b c. Beer-Lambert Law Introduction The Beer-Lambert law also called the Beer-Lambert-Bouguer law or simply Beers law is the linear relationship between absorbance and concentration of an absorber of electromagnetic radiation. The concentration of the sample was 60 which is very close to 626.
 Source: slideplayer.com
Source: slideplayer.com
Furthermore measurements can take place at one specific wavelength that is almost unique for bilirubin. Where you able to determine the concentration of the. The general Beer-Lambert law is usually written as. The Beer-Lambert Law implies that both the type and the concentration of the molecules are important in the process of radiation absorption. It has a molar absorptivity of 8400M-1cm-1.
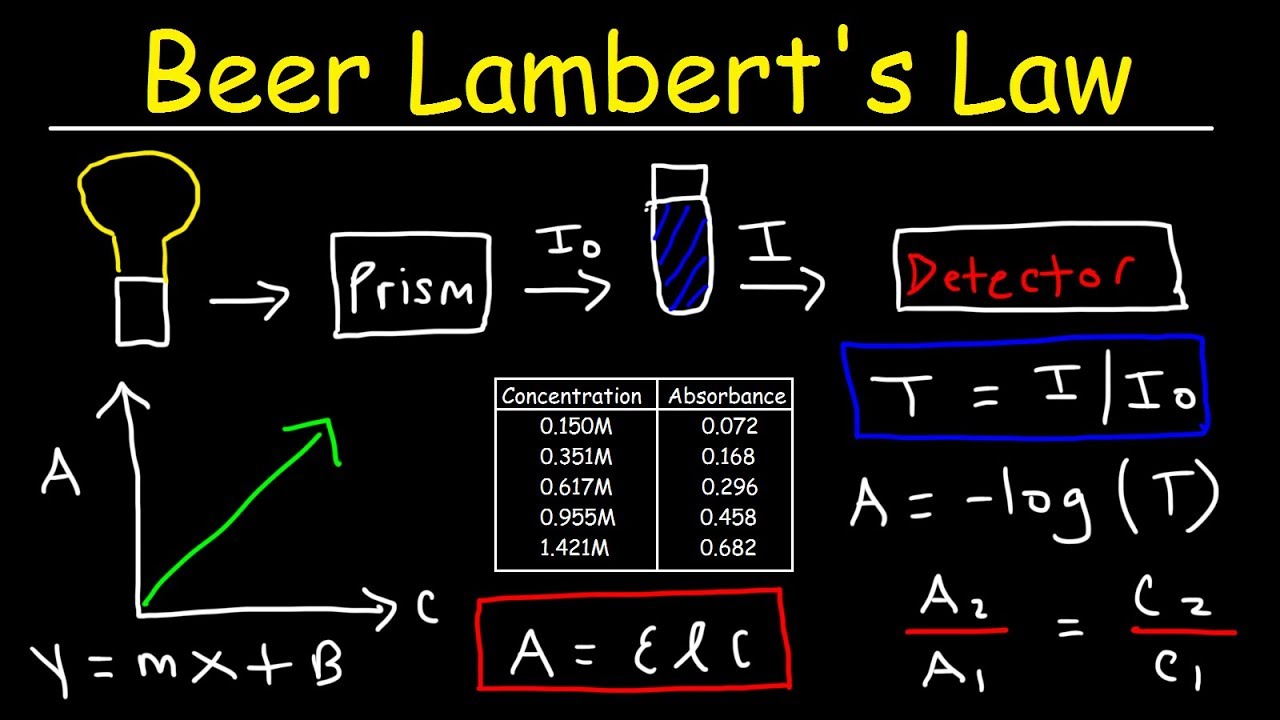 Source: youtube.com
Source: youtube.com
A absorbance a molar absorptivity in L mol cm b path length in cm and c is the concentration of the solution. The general Beer-Lambert law is usually written as. One has the data for the DMSO samples no allopurinol and the second has the data for allopurinol-treated samples. So c Aε l 054 64 10 3 05 Answer 0000168 M 2. A sample having a maximum absorbance value of 275nm.
 Source: youtube.com
Source: youtube.com
The law is often used in chemical analysis tests and is used to understand the physical optics of attenuation for neutrons photons and rarefied gas. Beer-Lambert Law and other calibration curves. Where A is the measured absorbance a λ is a wavelength-dependent absorptivity. Beer-Lambert law is the combination of 2 laws given by Johann Lambert and August Beer hence it came to be known as Beer-Lambert law. The Beer-Lambert Law implies that both the type and the concentration of the molecules are important in the process of radiation absorption.
 Source: youtube.com
Source: youtube.com
Spectrophotometry and the Beer-Lambert Law A classic example of this phenomenon is responsible for our perception of color. Lambert law states that absorbance and path length are directly proportional and it was stated by Johann Heinrich Lambert. Beers Law Example Calculation. Beer-Lambert law is the combination of 2 laws given by Johann Lambert and August Beer hence it came to be known as Beer-Lambert law. The important use of the Beer Lambert law is found in electromagnetic spectroscopy.
 Source: study.com
Source: study.com
The Beer-Lambert law is a linear relationship between the absorbance and the concentration molar absorption coefficient and optical coefficient of a solution. I I 0eμx I I 0 e μ x Where I is the intensity I0 is the initial intensity μ is the coefficient of absorption x is the depth in meter. Beer-Lambert Law Introduction The Beer-Lambert law also called the Beer-Lambert-Bouguer law or simply Beers law is the linear relationship between absorbance and concentration of an absorber of electromagnetic radiation. The Beer-Lambert law data analysis. Beer-Lambert Law for example a cuvette filled Beers law.
 Source: youtube.com
Source: youtube.com
The Beer-Lambert Law implies that both the type and the concentration of the molecules are important in the process of radiation absorption. Where you able to determine the concentration of the. According to the definition of absorbance. Beer Lambert Law Applications. There is no information in this law about the nature of light.
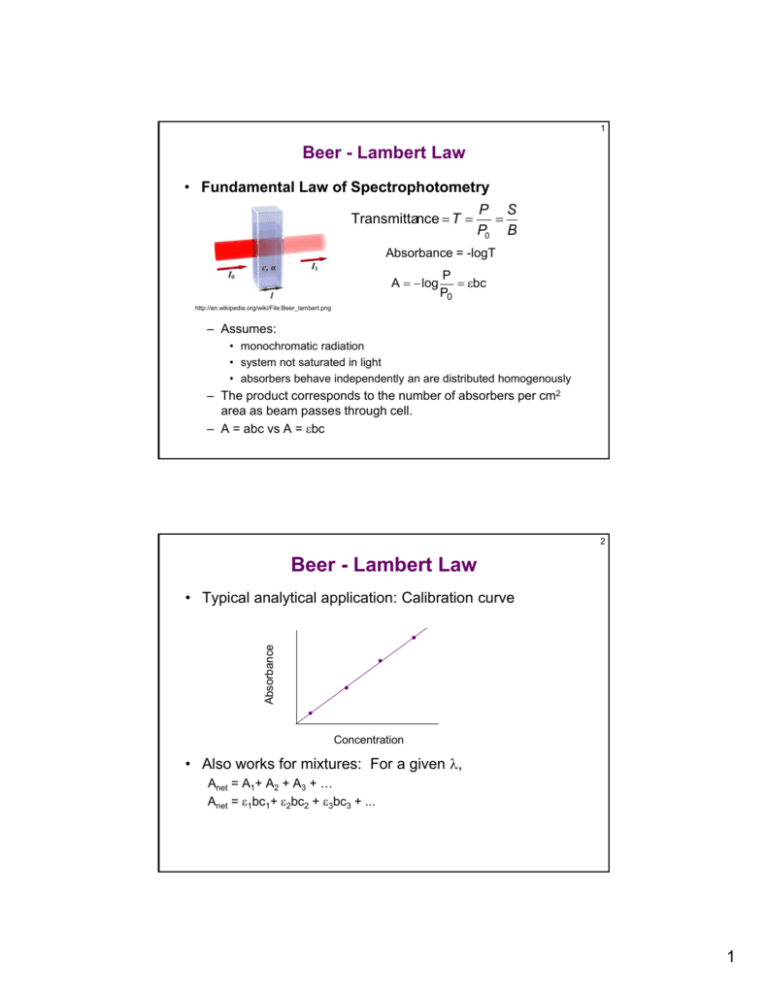 Source: studylib.net
Source: studylib.net
Section 4 details how to fix this problem and a toy example of calibration is developed in Section 5 to show how the two calibration curves are. Beer-Lambert Law Equation The Beer-Lambert law equation is as follows. Beers Law Example Calculation. The Beer-Lambert law is a linear relationship between the absorbance and the concentration molar absorption coefficient and optical coefficient of a solution. Thus log I t I o log 05 10 A 8 ϵ Then we obtain that ϵ 00376 Example 4 In Example 3 above what is the molar absorption coefficient if the molecular weight is 100.
 Source: slidetodoc.com
Source: slidetodoc.com
I I 0eμx I I 0 e μ x Where I is the intensity I0 is the initial intensity μ is the coefficient of absorption x is the depth in meter. Beers Law Example Calculation A sample is known to have a maximum absorbance value of 275 nm. Beers law in spectroscopy a relation concerning the absorption of. A absorbance a molar absorptivity in L mol cm b path length in cm and c is the concentration of the solution. The Beer-Lambert Law implies that both the type and the concentration of the molecules are important in the process of radiation absorption.
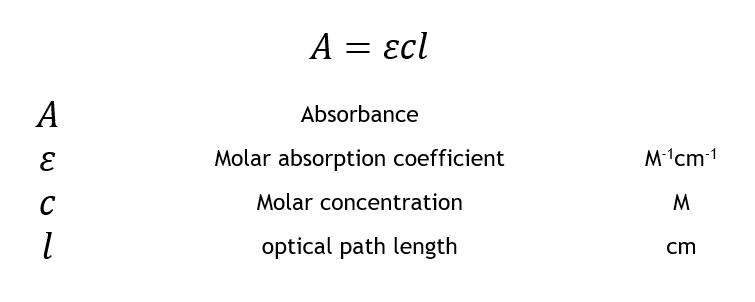 Source: geslab.net
Source: geslab.net
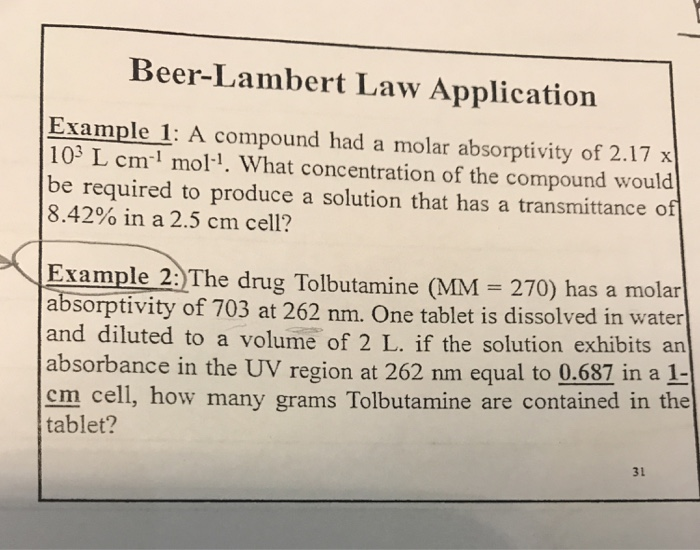
Where A is the measured absorbance a λ is a wavelength-dependent absorptivity. Spectrophotometry and the Beer-Lambert Law A classic example of this phenomenon is responsible for our perception of color. Lets suppose we have a tablet and we dont know which drug is present in it. Experiment C-28 Beer-Lambert law Ver 305 For example the absorbance of a solution with an unknown concentration is 072. A abc where.
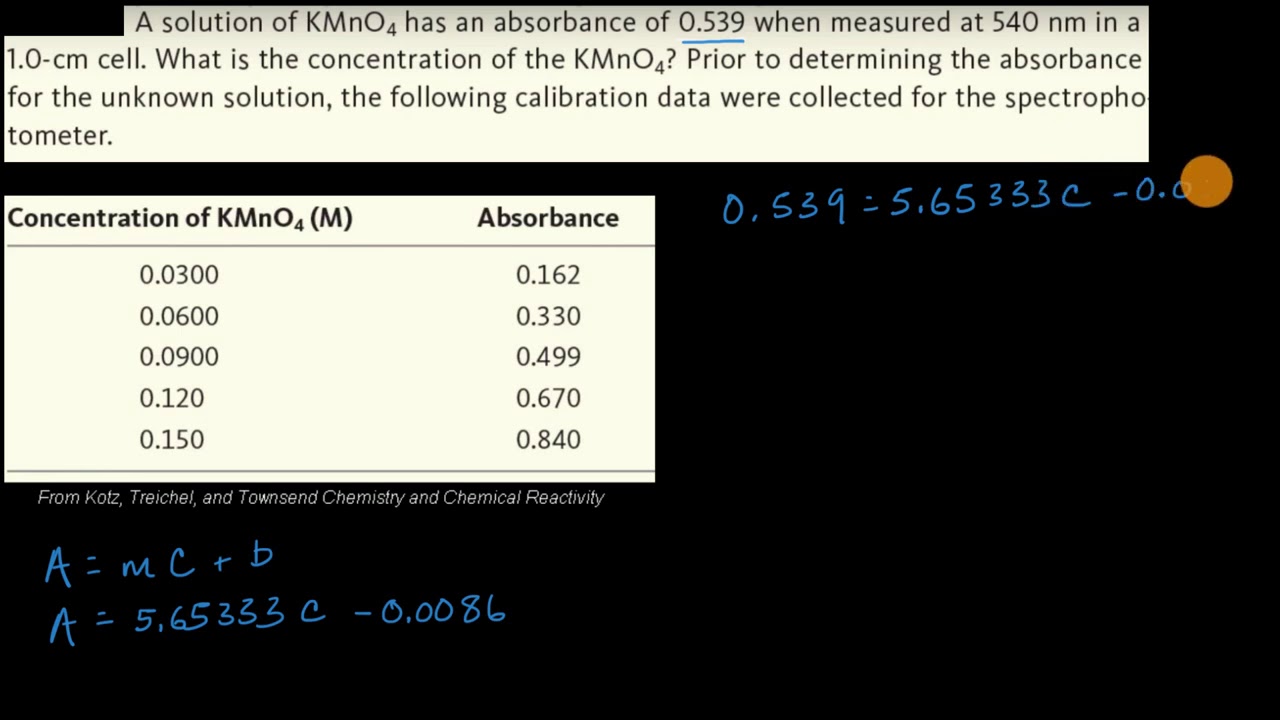 Source: khanacademy.org
Source: khanacademy.org
Experiment C-28 Beer-Lambert law Ver 305 For example the absorbance of a solution with an unknown concentration is 072. What is the concentration of the sample. Lets suppose we have a tablet and we dont know which drug is present in it. Now lets understand the applications of Beer Lambert law. To analyze the drugs for that lets take an example of a tablet.
 Source: slideshare.net
Source: slideshare.net
Beer-Lambert law is the combination of 2 laws given by Johann Lambert and August Beer hence it came to be known as Beer-Lambert law. A spectrophotometer finds A 070. Examples of Beer-Lambert Law Q1. 05 Feb 2022 by CLS4011-U Lab report data analysis guidance. Section 4 details how to fix this problem and a toy example of calibration is developed in Section 5 to show how the two calibration curves are.
 Source: study.com
Source: study.com
I I 0eμx I I 0 e μ x Where I is the intensity I0 is the initial intensity μ is the coefficient of absorption x is the depth in meter. The molar absorption coefficient is a sample dependent property and is a measure of how strong an absorber the sample is at a particular wavelength of light. A log 10 I 0 I The equation is rearranged to determine the relative loss of intensity 10A I0I 10-A II0. A solution of thickness 2. The law is often used in chemical analysis tests and is used to understand the physical optics of attenuation for neutrons photons and rarefied gas.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title beer lambert law example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






