Your Complete ionic equation example images are ready. Complete ionic equation example are a topic that is being searched for and liked by netizens now. You can Find and Download the Complete ionic equation example files here. Get all royalty-free images.
If you’re searching for complete ionic equation example images information linked to the complete ionic equation example keyword, you have come to the ideal site. Our website always gives you hints for refferencing the maximum quality video and image content, please kindly surf and find more informative video content and graphics that fit your interests.
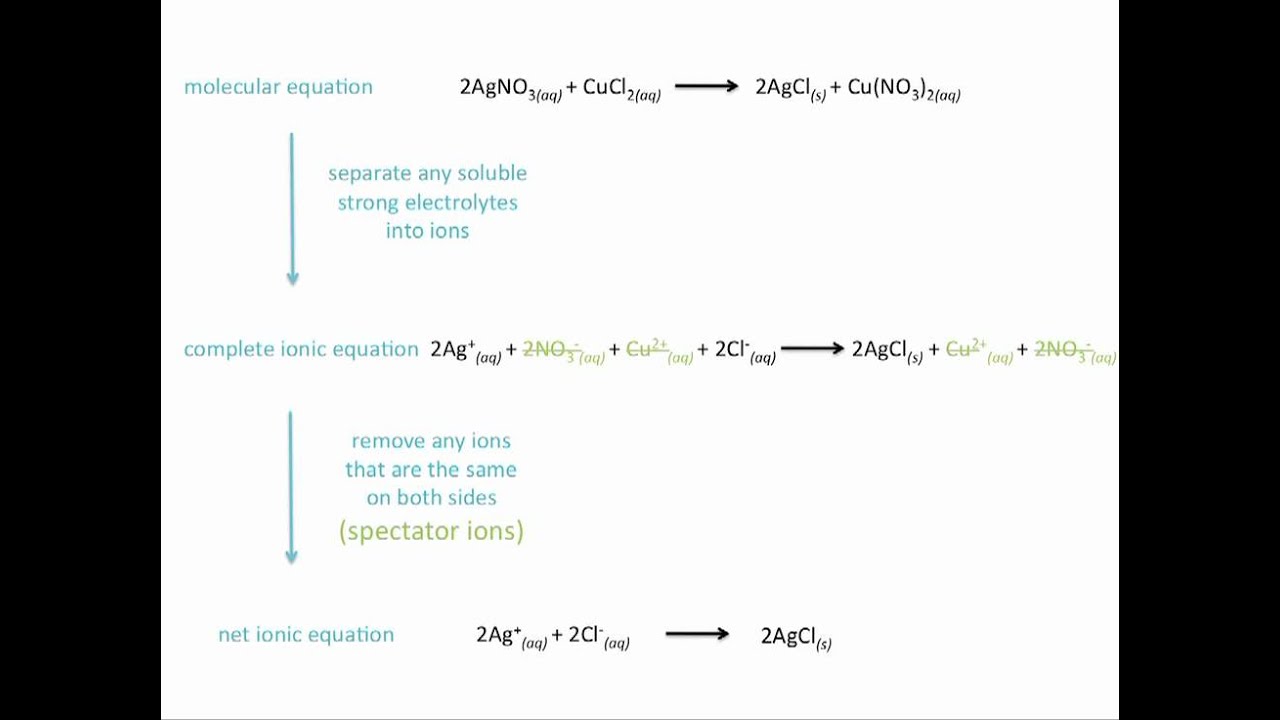
Complete Ionic Equation Example. Write the overall chemical equation the complete ionic equation and the net ionic equation for the reaction of aqueous barium nitrate with aqueous sodium phosphate to give solid barium phosphate and a solution of sodium nitrate. Basic lesson on molecular equations complete ionic equations and net ionic equations. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out. Compounds as separate ions.
 How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes From pt.pinterest.com
How To Write Ionic Equations Tutor Pace Science Chemistry Chemistry Class Chemistry Notes From pt.pinterest.com
The complete ionic equation indicates all of the dissociated ions in a chemical reaction. Use uppercase for the first character in the element and lowercase for the second character. Basic lesson on molecular equations complete ionic equations and net ionic equations. Key Difference Complete Ionic vs Net Ionic Equation Chemical reactions are interactions between chemical compounds to form new compounds or to rearrange their chemical structure. Ask unlimited questions and get expert help right away. The compounds that undergo a certain chemical reaction is called a reactant and what we get at the end is called the productA chemical equation is a representation of the.
Key Difference Complete Ionic vs Net Ionic Equation Chemical reactions are interactions between chemical compounds to form new compounds or to rearrange their chemical structure.
Answer 1 of 6. From the complete ionic equation we see that Na and Cl ions dont really participate in the reaction. For the neutralization reaction between any monoprotic strong acid and strong. Fe Au Co Br C O N F. Complete ionic equation. In a complete ionic equation all substances that are strong electrolytes are represented as ions.
 Source: pinterest.com
Source: pinterest.com
The word total can also be used as in total ionic equation or even simply total equation There is no standard term for this type of equation. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. Shows each of the. Example NaCl is an ionic salt When NaCl dissolves in water it breaks into a sodium ion a chloride ion NaCls Na aq Cl-aq aq aqueous dissolves in water s solid not dissolved in water Notice when they break into their. Write all the soluble reactants and products in their dissociated form to give the complete ionic equation.
 Source: pinterest.com
Source: pinterest.com
This precipitation reaction is described by the following equation. What is a complete ionic equation. Write all the soluble reactants and products in their dissociated form to give the complete ionic equation. Example NaCl is an ionic salt When NaCl dissolves in water it breaks into a sodium ion a chloride ion NaCls Na aq Cl-aq aq aqueous dissolves in water s solid not dissolved in water Notice when they break into their. Compounds as separate ions.
 Source: pinterest.com
Source: pinterest.com
This precipitation reaction is described by the following equation. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions. Write and balance the overall chemical equation. All of them are technically correct but each one is meant to show a different thing. The remaining equation is known as the.
 Source: pinterest.com
Source: pinterest.com
Write and balance the overall chemical equation. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. Write the overall chemical equation the complete ionic equation and the net ionic equation for the reaction of aqueous barium nitrate with aqueous sodium phosphate to give solid barium phosphate and a solution of sodium nitrate. Write all the soluble reactants and products in their dissociated form to give the complete ionic equation. 2 Na3PO4 aq 3 CaCl2 aq – 6 NaCl aq Ca3PO42 s.
 Source: pinterest.com
Source: pinterest.com
Write the balanced molecular equation for the reaction including the state of each substance. Ask unlimited questions and get expert help right away. Fe Au Co Br C O N F. The compounds that undergo a certain chemical reaction is called a reactant and what we get at the end is called the productA chemical equation is a representation of the. They are examples of what we call spectator ions.
 Source: ar.pinterest.com
Source: ar.pinterest.com
A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions. For the neutralization reaction between any monoprotic strong acid and strong. This means that we separate each molecule into its ion form. The reaction of potassium chloride and lead II nitrate. Na aq Cl aq Ag aq NO 3 aq AgCl s Na aq NO 3 aq.
 Source: pinterest.com
Source: pinterest.com
Basic lesson on molecular equations complete ionic equations and net ionic equations. Water is also not separated and it has a l written next to it. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. Molecular Complete Ionic and Net Ionic Equations How To Write A Net Ionic Equation Double Replacement. The complete ionic equation indicates all of the dissociated ions in a chemical reaction.
 Source: pt.pinterest.com
Source: pt.pinterest.com
Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. Overall complete ionic and net ionic equations. Write all the soluble reactants and products in their dissociated form to give the complete ionic equation. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions. And they are eliminated from complete ionic equation by crossing them out.
 Source: pinterest.com
Source: pinterest.com
Overall complete ionic and net ionic equations. Example NaCl is an ionic salt When NaCl dissolves in water it breaks into a sodium ion a chloride ion NaCls Na aq Cl-aq aq aqueous dissolves in water s solid not dissolved in water Notice when they break into their. The ions that are canceled out are called spectator ions. Molecular complete ionic and net ionic equations. 2 Na3PO4 aq 3 CaCl2 aq – 6 NaCl aq Ca3PO42 s.
 Source: pinterest.com
Source: pinterest.com
Use uppercase for the first character in the element and lowercase for the second character. What is a complete ionic equation. The reaction of potassium chloride and lead II nitrate. The balanced equation will be calculated along with the states complete ionic equation and the net ionic equation. The reaction of potassium chloride and lead II nitrate.
 Source: pinterest.com
Source: pinterest.com
That ionic equation is NOT the same as net ionic equation It is best to assume complete ionic equation is what is meant by ionic equation In other words the word net is always included when one means to identify the net ionic equation Here is an example of a mistake students sometimes make. The result of making all these replacements is the complete ionic equation. Na aq Cl aq Ag aq NO 3 aq AgCl s Na aq NO 3 aq. Write the balanced molecular equation for the reaction including the state of each substance. Ask unlimited questions and get expert help right away.
 Source: pinterest.com
Source: pinterest.com
The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially dont participate in the reaction of interest. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions. Example NaCl is an ionic salt When NaCl dissolves in water it breaks into a sodium ion a chloride ion NaCls Na aq Cl-aq aq aqueous dissolves in water s solid not dissolved in water Notice when they break into their. Molecular complete ionic and net ionic equations. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation.
 Source: pinterest.com
Source: pinterest.com
The ions that are canceled out are called spectator ions. Overall complete ionic and net ionic equations. The remaining equation is known as the. The complete ionic equation shows all the aqueous compounds broken up into ions. 2KCl aq PbNO 3 2.
 Source: pinterest.com
Source: pinterest.com
Lets use the reaction between sodium chloride and silver nitrate as an example. Enter an equation of an ionic chemical equation and press the Balance button. Ask unlimited questions and get expert help right away. Molecular Complete Ionic and Net Ionic Equations How To Write A Net Ionic Equation Double Replacement. These ions are known as spectator ions and they are eliminated from complete ionic equation by crossing them out.
 Source: pinterest.com
Source: pinterest.com
Complete ionic equation. The result of making all these replacements is the complete ionic equation. Writing Complete Ionic Equations When aqueous solutions of sodium phosphate and calcium chloride are mixed together an insoluble white solid forms. Convert the following balanced molecular equation into a complete ionic equation. The complete ionic equation indicates all of the dissociated ions in a chemical reaction.
 Source: pinterest.com
Source: pinterest.com
The remaining equation is known as the. 2KCl aq PbNO 3 2. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. All of them are technically correct but each one is meant to show a different thing. A net ionic equation shows only the chemical species that are involved in a reaction while a complete ionic equation also includes the spectator ions.
 Source: in.pinterest.com
Source: in.pinterest.com
Check your understanding of net ionic equations in this set of free practice questions designed for AP Chemistry students. Write the balanced molecular equation for the reaction including the state of each substance. Example NaCl is an ionic salt When NaCl dissolves in water it breaks into a sodium ion a chloride ion NaCls Na aq Cl-aq aq aqueous dissolves in water s solid not dissolved in water Notice when they break into their. Then cancel species that appear on both sides of the complete ionic equation to give the net ionic equation. The net ionic equation cancels out ions that appear on both sides of the reaction arrow because they essentially dont participate in the reaction of interest.
 Source: pinterest.com
Source: pinterest.com
Next we write the chemical equation as a complete ionic equation. Molecular complete ionic and net ionic equations. The complete ionic equation shows all the aqueous compounds broken up into ions. Molecular Complete Ionic and Net Ionic Equations How To Write A Net Ionic Equation Double Replacement. In a complete ionic equation all substances that are strong electrolytes are represented as ions.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title complete ionic equation example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






