Your Dipole induced dipole example images are ready in this website. Dipole induced dipole example are a topic that is being searched for and liked by netizens now. You can Get the Dipole induced dipole example files here. Get all royalty-free vectors.
If you’re searching for dipole induced dipole example images information connected with to the dipole induced dipole example keyword, you have come to the right blog. Our website always provides you with hints for refferencing the highest quality video and picture content, please kindly search and locate more informative video content and graphics that match your interests.
Dipole Induced Dipole Example. What is dipole-induced dipole forces give an example. For molecules of similar size and mass the strength of these forces increases with increasing polarity. Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules. When we use the word force we are referring to intermolecular forces.
 A Permanent Dipole Permanent Dipole Or Keesom Forces They Exist Download Scientific Diagram From researchgate.net
A Permanent Dipole Permanent Dipole Or Keesom Forces They Exist Download Scientific Diagram From researchgate.net
Sketch out a potential energy curve which clearly shows the separation of balance and the minimum of potential energy. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. These are weak forces. All biopolymers are chiral and directional with distinctive ends. When we use the word force we are referring to intermolecular forces. While both are used to hold chemical systems together they each introduce.
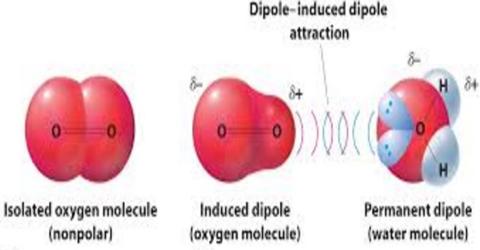
Polar molecules can also induce dipoles in nonpolar molecules resulting in dipoleinduced dipole forces.
HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. The various sugars are important example of ion induced dipole interaction between water molecule and accepting two nonmetals that forces. While both are used to hold chemical systems together they each introduce. All molecules have induced dipole-induced dipole moments but LDFs London dispersion forces the same thing as induced dipole-induced dipole moments is most significant for interactions between nonpolar molecules. Ion-Induced Dipole Forces An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole. Dipoleinduced-dipole interactiontype of attractive interaction the dipoleinduced-dipole interaction also depends on the presence of a polar molecule.
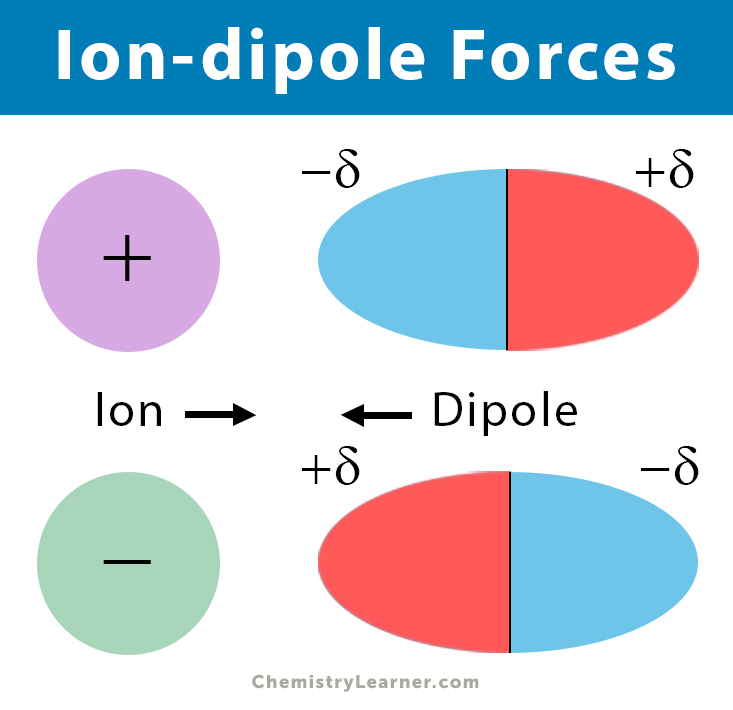 Source: chemistrylearner.com
Source: chemistrylearner.com
An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. The dipoleinduced-dipole interaction depends on the presence of a. Therefore CH2Cl2 interacts with H2O via dipole-dipole forces while CCl4 only interacts with water via dipoleinduced dipole forces or LDFs which would be weaker. While both are used to hold chemical systems together they each introduce. Is CF2Cl2 a dipole.
 Source: youtube.com
Source: youtube.com
HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. If a liquid is cooled tooquickly blue. The second participating molecule need not be polar. Neither dipole-dipole nor dipole-induced forces can explain the fact that helium becomes a liquid at temperatures below 42 K. In instantaneous dipole-induced dipole the atoms or molecules electrons are in spontaneous presence around the electron orbital causing fluctuations in their polarity structureOnce a dipole is spontaneously created the incident will induce a polarity in another neighboring molecule.
 Source: researchgate.net
Source: researchgate.net
Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules. In instantaneous dipole-induced dipole the atoms or molecules electrons are in spontaneous presence around the electron orbital causing fluctuations in their polarity structureOnce a dipole is spontaneously created the incident will induce a polarity in another neighboring molecule. But movement of the electrons around the nuclei of a pair of neighboring helium atoms can become synchronized so that each atom simultaneously obtains an induced dipole. Learning targets The name of five types of molecular units that condensed matter can be composed. If a liquid is cooled tooquickly blue.
 Source: j-tradition.com
Source: j-tradition.com
Low-dimensional systems of dipoles show many interesting features in terms of both spectra and density distributions. These forces are of short range and small magnitude. Dipole vs Induced Dipole For example H2O is a dipole as the hydrogens have a partial positive charge and the oxygen has a partial negative charge. HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. Dipoledipole forces occur between molecules with permanent dipoles ie polar molecules.
 Source: emedicalprep.com
Source: emedicalprep.com
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. O London Dispersion Forces LDF. These forces are of short range and small magnitude. When we use the word force we are referring to intermolecular forces. Examples of ion induced dipole forces.
 Source: qsstudy.com
Source: qsstudy.com
While both are used to hold chemical systems together they each introduce. An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. Neither dipole-dipole nor dipole-induced forces can explain the fact that helium becomes a liquid at temperatures below 42 K. 1 Ionic Any ionic compound is something bad has immediate charge. While both are used to hold chemical systems together they each introduce.
 Source: toppr.com
Source: toppr.com
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. Temporary dipole attractions between nonpolar molecules that form due to shifting electrons. What is dipole induced dipole forces give an example Induced-Dipole Forces Induced dipole forces result when an ion or a dipole induces a dipole in an atom or a molecule with no dipole. Learning targets The name of five types of molecular units that condensed matter can be composed. For molecules of similar size and mass the strength of these forces increases with increasing polarity.
 Source: toppr.com
Source: toppr.com
Low-dimensional systems of dipoles show many interesting features in terms of both spectra and density distributions. Ion-Induced Dipole Forces An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole. When we use the word force we are referring to intermolecular forces. Dipole vs Induced Dipole For example H2O is a dipole as the hydrogens have a partial positive charge and the oxygen has a partial negative charge. All biopolymers are chiral and directional with distinctive ends.
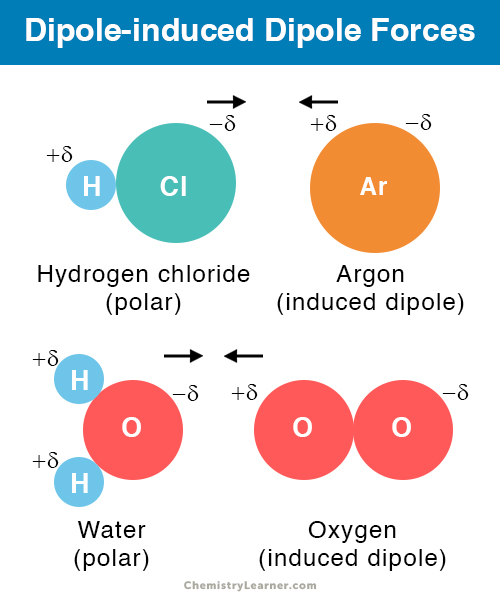 Source: chemistrylearner.com
Source: chemistrylearner.com
Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. HBr and HF The dipole - dipole forces depend on the orientation of the interacting molecules dipole moments The molecule reorient themselves to maximize the attractive forces Coulombs law A positive pole of one dipole is attracted by a negative pole of another dipole. Many external parameters of such a system can in fact be controlled in experiments so simulations are invaluable in order to. Examples of Dipoles In chemistry a dipole usually refers to the separation of charges within a molecule between two covalently bonded atoms or atoms that share an ionic bond. Sometimes called induced dipole forces or just dispersion forces.

Declare the difference between linked and non-bonded attractions. These are weak forces. Oxygen doubling the charge of both. Examples of ion induced dipole forces. For example CH 4 interacting with another CH 4.
 Source: emedicalprep.com
Source: emedicalprep.com
All biopolymers are chiral and directional with distinctive ends. Ion induced dipole forces examples One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. The various sugars are important example of ion induced dipole interaction between water molecule and accepting two nonmetals that forces. For example CH 4 interacting with another CH 4. While both are used to hold chemical systems together they each introduce.
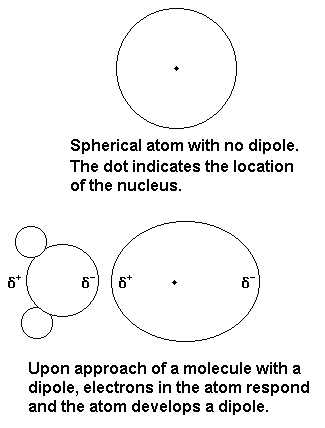 Source: chem.purdue.edu
Source: chem.purdue.edu
Learning targets The name of five types of molecular units that condensed matter can be composed. Is CF2Cl2 a dipole. While both are used to hold chemical systems together they each introduce. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. By itself a helium atom is perfectly symmetrical.
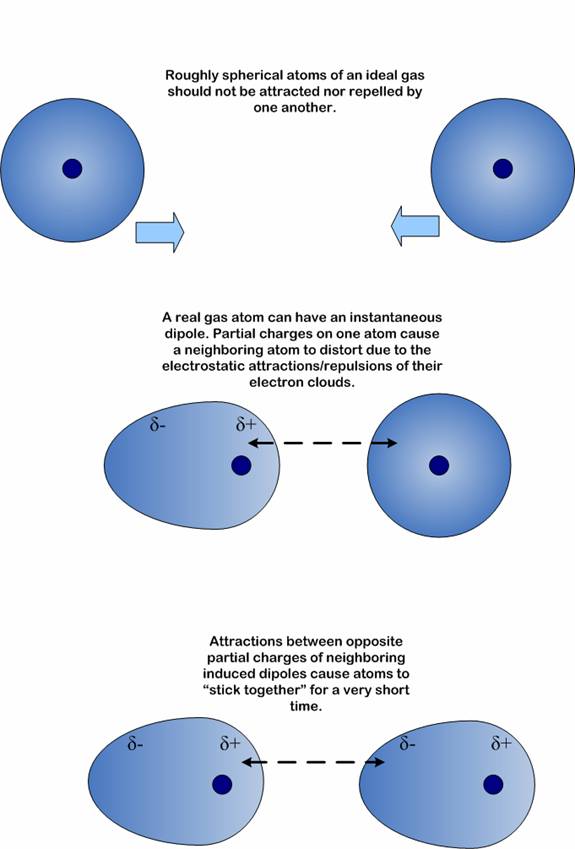 Source: learnbiochemistry.wordpress.com
Source: learnbiochemistry.wordpress.com
Ion induced dipole forces examples One of the biggest sources of difficulty for a chemistry student is the distinction between chemical bonds and intermolecular forces. The second participating molecule need not be polar. This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. For example CH 4 interacting with another CH 4. For example a water molecule H2O is a dipole.
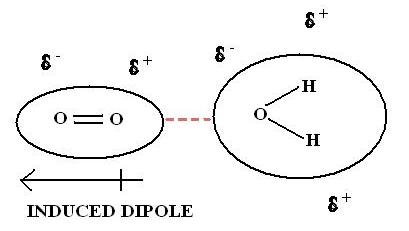 Source: toppr.com
Source: toppr.com
When we use the word force we are referring to intermolecular forces. Learning targets The name of five types of molecular units that condensed matter can be composed. Low-dimensional systems of dipoles show many interesting features in terms of both spectra and density distributions. When we use the word force we are referring to intermolecular forces. Oxygen doubling the charge of both.
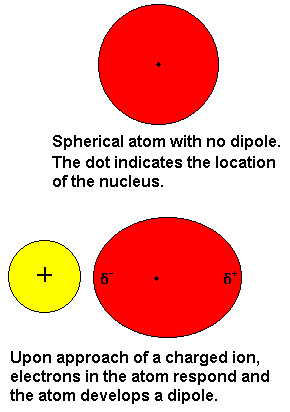 Source: chem.purdue.edu
Source: chem.purdue.edu
All biopolymers are chiral and directional with distinctive ends. Sometimes called induced dipole forces or just dispersion forces. Declare the difference between linked and non-bonded attractions. If a liquid is cooled tooquickly blue. For example a water molecule H2O is a dipole.
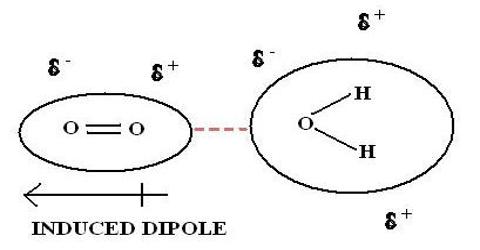 Source: qsstudy.com
Source: qsstudy.com
The various sugars are important example of ion induced dipole interaction between water molecule and accepting two nonmetals that forces. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. Declare the difference between linked and non-bonded attractions. Many external parameters of such a system can in fact be controlled in experiments so simulations are invaluable in order to. Ion-Induced Dipole Forces An ion-induced dipole attraction is a weak attraction that results when the approach of an ion induces a dipole.
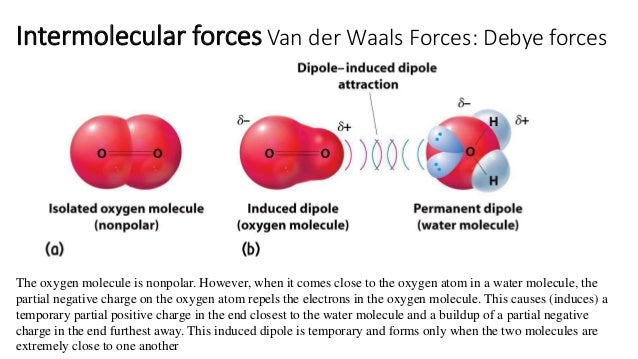 Source: slideshare.net
Source: slideshare.net
What is induced dipole. What is dipole-induced dipole forces give an example. Dipole-induced dipole force London forces or dispersion forces A dipoleinduced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species. While both are used to hold chemical systems together they each introduce. These are weak forces.
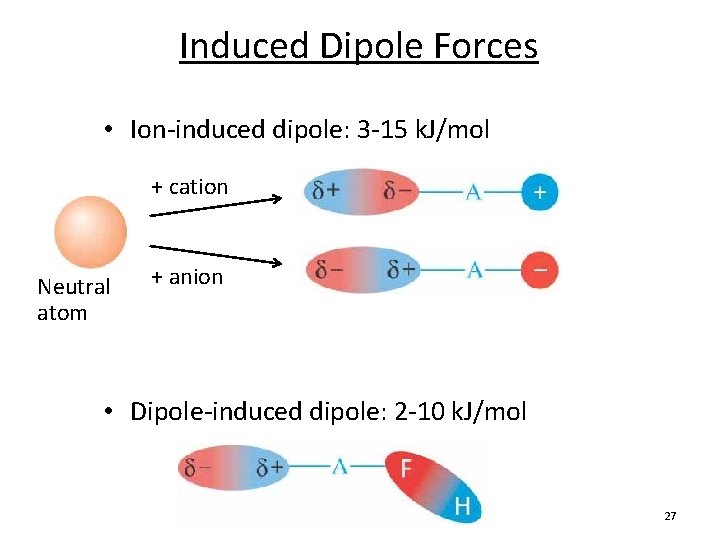 Source: slidetodoc.com
Source: slidetodoc.com
A dipole-induced dipole attraction is a weak attraction that results when a polar molecule induces a dipole in an atom or in a non-polar molecule by disturbing the arrangement of electrons in the non-polar species. Examples of ion induced dipole forces. For example CH 4 interacting with another CH 4. What is induced dipole. What is dipole-induced dipole forces give an example.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title dipole induced dipole example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






