Your Double displacement reaction examples images are available in this site. Double displacement reaction examples are a topic that is being searched for and liked by netizens now. You can Find and Download the Double displacement reaction examples files here. Download all royalty-free vectors.
If you’re looking for double displacement reaction examples images information related to the double displacement reaction examples keyword, you have pay a visit to the right site. Our website frequently provides you with suggestions for refferencing the highest quality video and picture content, please kindly surf and find more informative video content and graphics that match your interests.
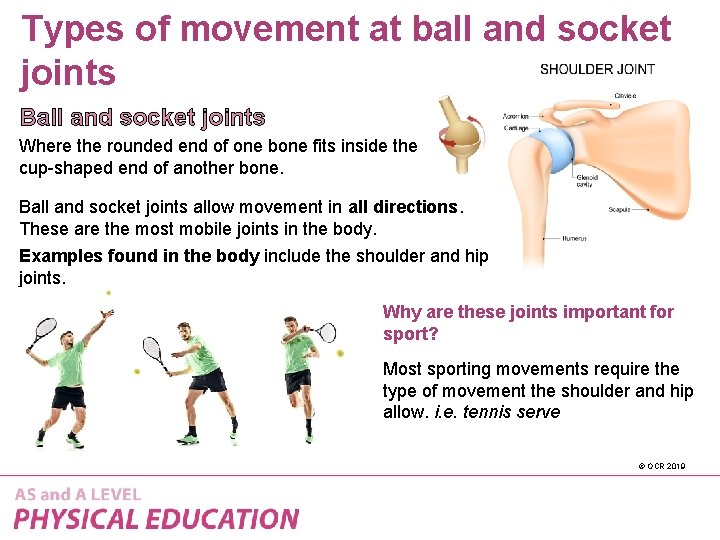
Double Displacement Reaction Examples. A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. 6If you combine vinegar and baking soda for a chemical volcano or milk with baking powder in a recipe you experience a double displacement or metathesis. For example on mixing a solution of barium chloride with sodium sulphate a white precipitate of barium sulphate is immediately formed. Reaction of iron nails with copper sulphate solution.
 Stoichiometry Quiz Chemistry Class Teaching Science Chemistry From pinterest.com
Stoichiometry Quiz Chemistry Class Teaching Science Chemistry From pinterest.com
Fe O2 H2O Fe2O3. Copper sulphate with hydrogen sulphide CuS0 4 aq H 2 S g CuS s H 2 SO 4 aq Copper sulphate react with hydrogen sulphide to form sulphuric acid. Pb NO32 aq 2NaI aq PbI2 s 2NaNO3 aq In this reaction lead nitrate reacts with sodium iodide to form lead iodide and sodium nitrate. In double displacement reaction there is displacement of 2 substances to form 2 new products. These reactions are ionic in nature. Both metals and non-metals take part in displacement reactions.
Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound.
A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. BaCl2aq Na2SO4aq BaSO4s 2 NaCl aq Click to see full answer Consequently what is an example of a double replacement reaction. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. The solvent for a double replacement reaction is usually water and the reactants and products are usually ionic compoundsbut they can also be acids or bases. CHEMICAL REACTIONS AND EQUAT. Direct Combination Synthesis Reaction.
 Source: pinterest.com
Source: pinterest.com
Metathesis Double Displacement Reaction. When Sodium Sulphate solution is mixed with Barium Chloride solution It forms Barium Sulphate and Sodium Chloride solution. Fe O2 H2O Fe2O3. These reactions are ionic in nature. Double displacement reaction examples Example1 Li2 THEN4 aq BaCl2 aq 2LiCl aq BaSO4 s If we want to remove barium from a solution we can just add lithium sulfate.
 Source: pinterest.com
Source: pinterest.com
Other everyday examples include formation of verdigris on copper and tarnishing of silver. In preparation of silver chloride B. Direct Combination Synthesis Reaction. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. In easy language.
 Source: pinterest.com
Source: pinterest.com
Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. Both metals and non-metals take part in displacement reactions. In preparation of silver chloride B. 2KI aq Pb NO 3 2 aq 2KNO 3 aq PbI 2 s Ques. When Sodium Sulphate solution is mixed with Barium Chloride solution It forms Barium Sulphate and Sodium Chloride solution.
 Source: pinterest.com
Source: pinterest.com
Examples of double displacement reaction. The type of reactions taking place in each of the following cases are. Examples of Double Displacement Reactions For our first example well look at the reaction between Li 2 SO 4 and BaCl2. Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions.
 Source: pinterest.com
Source: pinterest.com
In double displacement reaction there is displacement of 2 substances to form 2 new products. H 2 SO 4 2LiOH Li 2 SO 4 2H 2 O. For example on mixing a solution of barium chloride with sodium sulphate a white precipitate of barium sulphate is immediately formed. Silver nitrate reaction sodium chloride AgNO 3 aq NaCl aq AgCl s NaNO 3 aq Uses. When Sodium Sulphate solution is mixed with Barium Chloride solution It forms Barium Sulphate and Sodium Chloride solution.
 Source: pl.pinterest.com
Source: pl.pinterest.com
HCl NaOH NaCl H 2 O. This is an example of an oxidation reaction. Some precipitation reactions acid-base reactions and alkylation reactions are good examples of double displacement reactions. Give an example of a double displacement reaction other than the one given in Activity 110Class10Subject. Direct Combination Synthesis Reaction.
 Source: pinterest.com
Source: pinterest.com
BaCl2aq Na2SO4aq BaSO4s 2 NaCl aq Click to see full answer Consequently what is an example of a double replacement reaction. The subscript of SO4 is 1. 6If you combine vinegar and baking soda for a chemical volcano or milk with baking powder in a recipe you experience a double displacement or metathesis. A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. In double displacement reaction there is displacement of 2 substances to form 2 new products.
 Source: pinterest.com
Source: pinterest.com
For example on mixing a solution of barium chloride with sodium sulphate a white precipitate of barium sulphate is immediately formed. HCl NaOH NaCl H 2 O. This is an example of an oxidation reaction. Pb NO 3 2 2NaCl 2NaNO 3 PbCl 2. In double displacement reaction there is displacement of 2 substances to form 2 new products.
 Source: pinterest.com
Source: pinterest.com
It is a displacement reaction. This is an example of an oxidation reaction. In easy language. Zn s 2AgNO 3 aq Zn NO 3 2 aq 2Ag s The given reaction is a double displacement reaction. Example of double displacement reaction Reaction between silver nitrate and sodium chloride is an example of double displacement reaction.
 Source: pinterest.com
Source: pinterest.com
Examples of double displacement reaction. Single Displacement Substitution Reaction. CHEMICAL REACTIONS AND EQUAT. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. Examples of Double Displacement Reactions For our first example well look at the reaction between Li 2 SO 4 and BaCl2.
 Source: pinterest.com
Source: pinterest.com
A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. Direct Combination Synthesis Reaction. Examples of double-displacement reactions 5. Double displacement reaction examples Example1 Li2 THEN4 aq BaCl2 aq 2LiCl aq BaSO4 s If we want to remove barium from a solution we can just add lithium sulfate.
 Source: pinterest.com
Source: pinterest.com
Double Displacement Reaction example A. Double Displacement Reaction example A. Examples of Double Displacement Reactions Precipitation Reactions Here two compounds react with each other to form a solid in case the maximum solubility of the solution is reached. The type of reactions taking place in each of the following cases are. If we swap the anions or cations we get as our products and.
 Source: pinterest.com
Source: pinterest.com
Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. A B - C D - A D - C B - In this type of reaction the positive-charged cations and the negative-charged anions of the reactants both trade places double displacement to form two new products. AgNO 3 NaCl AgCl NaNO 3. CHEMICAL REACTIONS AND EQUAT. Examples of double-displacement reactions 5.
 Source: pinterest.com
Source: pinterest.com
Direct Combination Synthesis Reaction. It is a displacement reaction. Both metals and non-metals take part in displacement reactions. Direct Combination Synthesis Reaction. Pb NO 3 2 2NaCl 2NaNO 3 PbCl 2.
 Source: pinterest.com
Source: pinterest.com
BaCl2aq Na2SO4aq BaSO4s 2 NaCl aq Click to see full answer Consequently what is an example of a double replacement reaction. HCl NaOH NaCl H 2 O. Here is the double displacement model. Metathesis Double Displacement Reaction. Direct Combination Synthesis Reaction.
 Source: pinterest.com
Source: pinterest.com
AgNO 3 HCl AgCl HNO 3. A B - C D - A D - C B - In this type of reaction the positive-charged cations and the negative-charged anions of the reactants both trade places double displacement to form two new products. AgNO3 NaCl AgCl NaNO3. BaCl2aq Na2SO4aq BaSO4s 2 NaCl aq Click to see full answer Consequently what is an example of a double replacement reaction. Here is the chemical equation for the rusting of iron.
 Source: pinterest.com
Source: pinterest.com
In this example the cations are and and the anions are and. A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. Pb NO32 aq 2NaI aq PbI2 s 2NaNO3 aq In this reaction lead nitrate reacts with sodium iodide to form lead iodide and sodium nitrate. Here is the double displacement model. Examples of Double Displacement Reactions Precipitation Reactions Here two compounds react with each other to form a solid in case the maximum solubility of the solution is reached.
 Source: pinterest.com
Source: pinterest.com
Examples of Double Displacement Reactions For our first example well look at the reaction between Li 2 SO 4 and BaCl2. A double displacement reaction is a type of chemical reaction where two compounds react and positive ions cation and the negative ions anion of the two reactants switch places forming two new compounds or products. Fe O2 H2O Fe2O3. What is double displacement reaction explain with an example. SO4 is a polyatomic ion so we will treat this as one anion.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title double displacement reaction examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.