Your Dry cell battery examples images are available. Dry cell battery examples are a topic that is being searched for and liked by netizens now. You can Get the Dry cell battery examples files here. Find and Download all free images.
If you’re looking for dry cell battery examples pictures information linked to the dry cell battery examples keyword, you have pay a visit to the ideal site. Our site always gives you suggestions for seeing the maximum quality video and image content, please kindly search and find more enlightening video content and graphics that match your interests.
Dry Cell Battery Examples. One example of a dry cell is anode which is a zinc metal. In such cells a zinc container acts as the anode and a carbon rod acts as the cathode. A primary cell cannot be recharged because the internal chemical reaction cannot be restored. Zinc-carbon cell is the most common dry cell and is also called Leclanche cell.
 Structure Types And Working Of Dry Cell Utmel From utmel.com
Structure Types And Working Of Dry Cell Utmel From utmel.com
They are small light and do not leak chemicals making them easy to handle. A dry cell battery is full of solid or paste-like electrolytes. Assign the reactions to the correct electrode cathode or anode. This ScienceStruck post provides the history definition composition uses and recycling process of the dry cell battery. One example of a dry cell is anode which is a zinc metal. In todays power savvy world dry cell is one of many types of electrochemical cells available for consumer use but it was a great innovation when it was inventedWet-cell batteries which came first were normally delicate glass holders.
AAA AA C and D-cell batteries are all examples of dry cells as are the 9-volt batteries you use in smoke alarms.
2MnO 2 H 2 Mn 2 O 3 H 2 O. Dry cell batteries consist of a sealed chamber containing an anode cathode and paste-like electrolyte. Common examples of dry-cell batteries include zinc-carbonbatteries and alkaline batteriesA dry cell is a type of electric battery commonly used for portable with electrical devices. Electrochemical batteries are classified into 4 broad categories. It was invented in 1886 by German scientist Carl Gassner after development of wet zinc-carbon batteries by Georges Leclanche in 1886. A secondary cell or storage cell can be recharged because its chemical reaction is reversible.
 Source: utmel.com
Source: utmel.com
The common AA and AAA batteries found in wall clocks television remotes and other electrical devices are examples of these disposable batteries. A dry cell battery is full of solid or paste-like electrolytes. The common AA and AAA batteries found in wall clocks television remotes and other electrical devices are examples of these disposable batteries. Examples of dry cell primary cells include zinc-carbon and alkaline cells. Called alsoRN dry battery.
 Source: youtube.com
Source: youtube.com
The overall reaction is Zn 2MnO 2 2NH 4 Cl Mn 2 O 3. A dry cell battery is full of solid or paste-like electrolytes. However dry cells are much more difficult to manufacture than wet cells. AAA AA C and D-cell batteries are all examples of dry cells as are the 9-volt batteries you use in smoke alarms. Dry-cell battery stores energy in an immobilized electrolyte paste which minimizes the need for water.
 Source: sciencestruck.com
Source: sciencestruck.com
Noun a voltaic cell whose contents are not spillable. Dry cells have a moist electrolyte that cannot be spilled. This is the difference between dry cell and wet cell. In todays power savvy world dry cell is one of many types of electrochemical cells available for consumer use but it was a great innovation when it was inventedWet-cell batteries which came first were normally delicate glass holders. One example of a dry cell is anode which is a zinc metal.
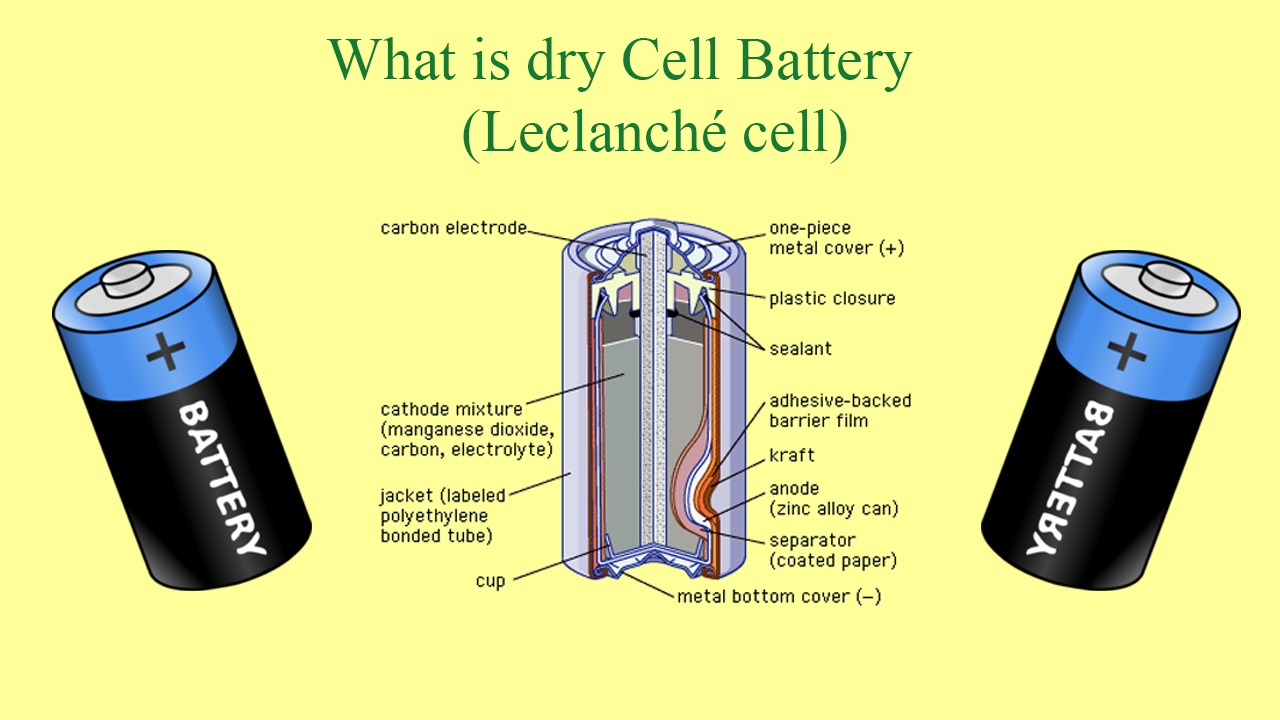 Source: youtube.com
Source: youtube.com
A primary cell or battery is one that cannot easily be recharged after one use and are discarded following discharge. The reactions that occur in this battery are shown below. However dry cells are much more difficult to manufacture than wet cells. Most primary cells utilize electrolytes that are contained within absorbent material or a separator ie. A secondary cell or storage cell can be recharged because its chemical reaction is reversible.
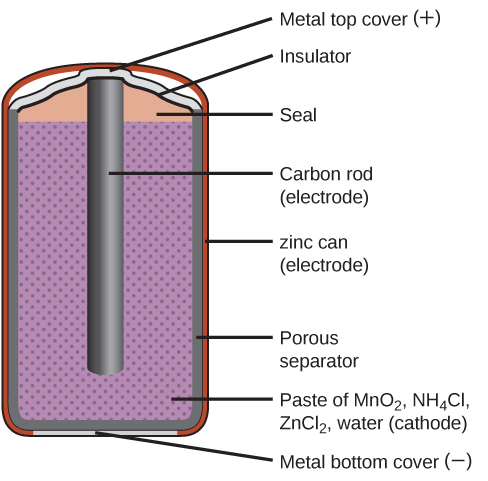 Source: opentextbc.ca
Source: opentextbc.ca
In todays power savvy world dry cell is one of many types of electrochemical cells available for consumer use but it was a great innovation when it was inventedWet-cell batteries which came first were normally delicate glass holders. Examples of secondary cells include lithium-ion and nickel-metal hydride cells Dry cell batteries are more convenient for mobile applications and now often offer higher performance. A primary cell or battery is one that cannot easily be recharged after one use and are discarded following discharge. Dry cells have a moist electrolyte that cannot be spilled. Assign the reactions to the correct electrode cathode or anode.
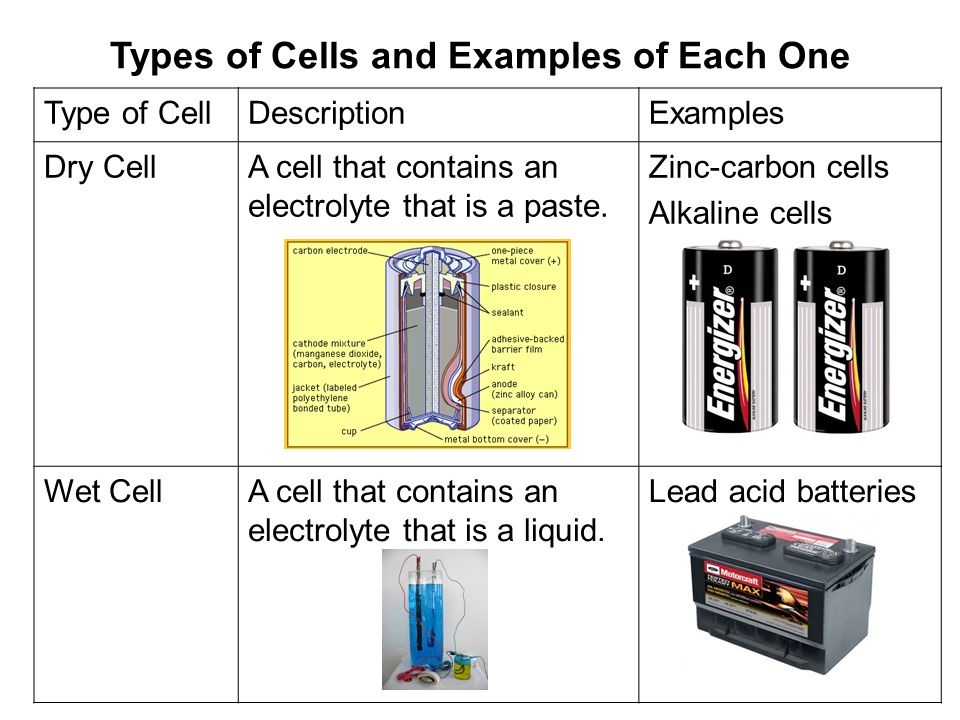 Source: slideplayer.com
Source: slideplayer.com
Whether a battery may be recharged or not depends on the cells used to make up the battery. One example of a dry cell is anode which is a zinc metal. In such cells a zinc container acts as the anode and a carbon rod acts as the cathode. Called alsoRN dry battery. A dry cell is a type of electric battery commonly used for portable with electrical devices.
 Source: elprocus.com
Source: elprocus.com
AAA AA C and D-cell batteries are all examples of dry cells as are the 9-volt batteries you use in smoke alarms. This process is irreversible which means that. This cell was first invented by French engineer Georges Leclanche in the year 1866. ZNCl 2 2NH 3 ZnNH 3 2 Cl 2. Examples of dry cell primary cells include zinc-carbon and alkaline cells.
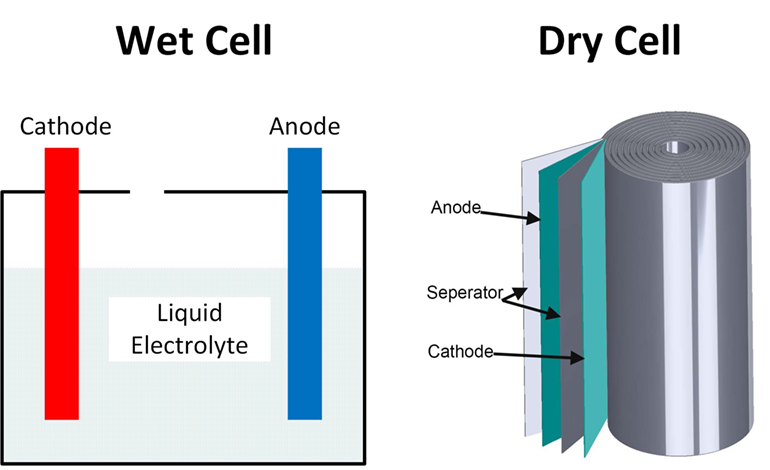 Source: batterypowertips.com
Source: batterypowertips.com
The dry cell is by far the most common type of battery used in several electronic devices such as TV remotes watches and many other devices. 2MnO 2 H 2 Mn 2 O 3 H 2 O. A battery is a gadget comprised of one or more electrochemical cells that convert the stored chemical energy into electrical energy. A common primary battery is the dry cell also known as a zinc-carbon battery. The lead-acid or nickel-cadmium battery is the advanced version of dry cell.
![]() Source: electroniclinic.com
Source: electroniclinic.com
Electrochemical batteries are classified into 4 broad categories. An example of a primary battery is the dry cell the household battery that commonly used to power TV remotes clocks and other devices. A primary cell or battery is one that cannot easily be recharged after one use and are discarded following discharge. Dry cells have a higher energy density compared to wet cells. This process is irreversible which means that.

In todays power savvy world dry cell is one of many types of electrochemical cells available for consumer use but it was a great innovation when it was inventedWet-cell batteries which came first were normally delicate glass holders. However dry cells are much more difficult to manufacture than wet cells. A dry cell battery is full of solid or paste-like electrolytes. A common primary battery is the dry cell also known as a zinc-carbon battery. In this battery type zinc acts as the anode and carbon acts as the cathode where the electrolyte is composed of a salt-base.
 Source: researchgate.net
Source: researchgate.net
An example of a primary battery is the dry cell the household battery that commonly used to power TV remotes clocks and other devices. The electrolyte in this cell battery contains very little moisture to allow the passage of current through it. Dry cells have a higher energy density compared to wet cells. Assign the reactions to the correct electrode cathode or anode. They are small light and do not leak chemicals making them easy to handle.
 Source: electrical4u.com
Source: electrical4u.com
As in the dry cell Zn is the anode. The common AA and AAA batteries found in wall clocks television remotes and other electrical devices are examples of these disposable batteries. AAA AA C and D-cell batteries are all examples of dry cells as are the 9-volt batteries you use in smoke alarms. A secondary cell or storage cell can be recharged because its chemical reaction is reversible. Dry cells have a moist electrolyte that cannot be spilled.
 Source: electricalmag.com
Source: electricalmag.com
Dry cells have a higher energy density compared to wet cells. Whether a battery may be recharged or not depends on the cells used to make up the battery. A wet cell battery is full of liquid electrolytes. Primary cells include the Daniell cell Dry cell and Mercury cell. This ScienceStruck post provides the history definition composition uses and recycling process of the dry cell battery.
 Source: shutterstock.com
Source: shutterstock.com
One example of a dry cell is anode which is a zinc metal. A wet cell battery is full of liquid electrolytes. A battery is a gadget comprised of one or more electrochemical cells that convert the stored chemical energy into electrical energy. The dry cell is by far the most common type of battery used in several electronic devices such as TV remotes watches and many other devices. As in the dry cell Zn is the anode.
 Source: pinterest.com
Source: pinterest.com
The overall reaction is Zn 2MnO 2 2NH 4 Cl Mn 2 O 3. In todays power savvy world dry cell is one of many types of electrochemical cells available for consumer use but it was a great innovation when it was inventedWet-cell batteries which came first were normally delicate glass holders. However dry cells are much more difficult to manufacture than wet cells. A dry cell is the simplest form of electricity-producing source. Ad Free Shipping Available.
 Source: britannica.com
Source: britannica.com
A primary cell or battery is one that cannot easily be recharged after one use and are discarded following discharge. A dry cell battery is full of solid or paste-like electrolytes. A nother example of a common primary battery is the alkaline battery. ZNCl 2 2NH 3 ZnNH 3 2 Cl 2. A dry cell is a type of electric battery commonly used for portable with electrical devices.
 Source: csun.edu
Source: csun.edu
A wet cell battery is full of liquid electrolytes. Ad Free Shipping Available. Most primary cells utilize electrolytes that are contained within absorbent material or a separator ie. This battery type degrades over time as it starts to leak due to an oxidation of the zinc. Dry cell batteries consist of a sealed chamber containing an anode cathode and paste-like electrolyte.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
A primary cell or battery is one that cannot easily be recharged after one use and are discarded following discharge. Whether a battery may be recharged or not depends on the cells used to make up the battery. As in the dry cell Zn is the anode. Zinc-carbon cell is the most common dry cell and is also called Leclanche cell. A primary cell cannot be recharged because the internal chemical reaction cannot be restored.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title dry cell battery examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






