Your Example of thermal decomposition reaction images are available. Example of thermal decomposition reaction are a topic that is being searched for and liked by netizens now. You can Find and Download the Example of thermal decomposition reaction files here. Find and Download all free vectors.
If you’re looking for example of thermal decomposition reaction pictures information connected with to the example of thermal decomposition reaction keyword, you have visit the right site. Our website always gives you hints for viewing the maximum quality video and picture content, please kindly search and find more enlightening video content and images that fit your interests.
Example Of Thermal Decomposition Reaction. Ii Thermal decomposition reactions form one of the steps in extraction of metals. Decomposition is defined as the chemical reaction in which a single compound gives two or more simple substances. This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source. 2Fe OH 3 heatFe 2 O 3 3H 2 O Ques.
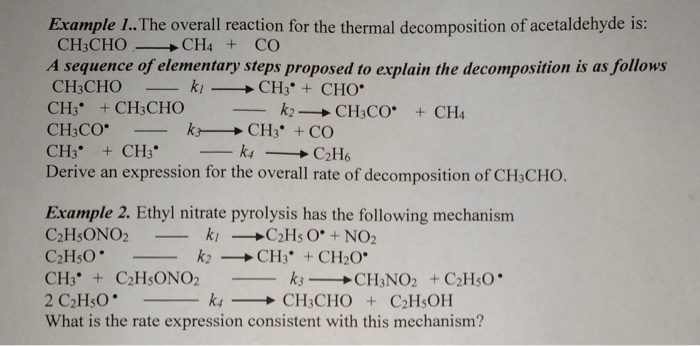
For example sodium hydrogen carbonate also called baking soda thermally decomposes when you bake muffins. The examples of thermal decomposition include. Thermal decomposition - such reactions are usually. Example 5 Electrolysis of water The breaking down of H 2 O into H 2 and O 2 by passing electricity is known as electrolysis of water. Examples of thermal dissociation are. Thermal decomposition is a chemical change with new substances being produced.
The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction.
The gas produced in this. Chemical reactions and equations. A decomposition reaction has following mechanism. H₂O₂ H₂ O₂ Classification of Decomposition Reaction. What are the uses of decomposition reactions. Zinc carbonate on heating decomposes to form Zinc oxide and carbon dioxide.

Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce. If a test tube of CuCO 3 is heated in a Bunsen burner the solid powder jumps around as molecules of carbon dioxide gas are released. Thermal composition reaction or thermolysis is the decomposition by means of heat. This is called thermal decomposition because heat energy is absorbed from the surroundings such as from a heat source. CuSO4 CuO SO3 H2O SO3 H2SO4.
 Source: drgpinstitute.in
Source: drgpinstitute.in
What are the unique characteristics of a decomposition reaction. CH3Br – CH3 Br CH3CH3 – CH3 CH3 Thermal decomposition implies a multi-step reaction with a complicated mixture of products. Usually only one bond breaks and the reaction is reversible. The gas produced in this. An example is when baking soda sodium bicarbonate is heated 2N aH CO3s CO2g H 2Og N a2CO3s Here is a video discussion of this reaction.
 Source: learneasytutorial.com
Source: learneasytutorial.com
If a test tube of CuCO 3 is heated in a Bunsen burner the solid powder jumps around as molecules of carbon dioxide gas are released. The process is not reversible. Write relevant balanced chemical equations also. If a test tube of CuCO 3 is heated in a Bunsen burner the solid powder jumps around as molecules of carbon dioxide gas are released. CaCO 3 CaO CO 2 Combustion.
 Source: icsehelp.com
Source: icsehelp.com
Example 5 Electrolysis of water The breaking down of H 2 O into H 2 and O 2 by passing electricity is known as electrolysis of water. Calcium carbonate is heated to decompose into calcium oxide and carbon dioxide. An example of t Continue Reading Related Answer Aman Sharma former Student. The equation is represented below. Carbonates decompose into carbon dioxide and an oxide.
 Source: brainly.in
Source: brainly.in
Thermal decomposition is represented by a general formula format. The gas produced in this. Decomposition carried out by heating is called thermal decomposition reaction. Thermal decomposition reaction is also known as thermolysis. Thermal decomposition is represented by a general formula format.

An example of a thermal decomposition reaction application is in the manufacture of quick lime calcium oxide CaO also called burnt lime. There are three types of decomposition reactions. The equation is represented below. Ii Thermal decomposition reactions form one of the steps in extraction of metals. A great example of a thermal decomposition reaction which is the type of decomposition that occurs with the addition of heat is the breakdown of copper carbonate CuCO 3.
 Source: slideplayer.com
Source: slideplayer.com
Thermal decomposition reaction is also known as thermolysis. Thermal decomposition is represented by a general formula format. Thermal Decomposition Reaction. When we eat foods like wheat rice. CH3Br – CH3 Br CH3CH3 – CH3 CH3 Thermal decomposition implies a multi-step reaction with a complicated mixture of products.
 Source: brainly.in
Source: brainly.in
Thermal decomposition is a chemical change with new substances being produced. The gas produced in this. 0 0 Similar questions. For example Zinc carbonate the naturally occurring ore of zinc is first decomposed to give zinc oxide and then reduced to obtain zinc metal ie Decomposition Reactions in our body. For example sodium hydrogen carbonate also called baking soda thermally decomposes when you bake muffins.
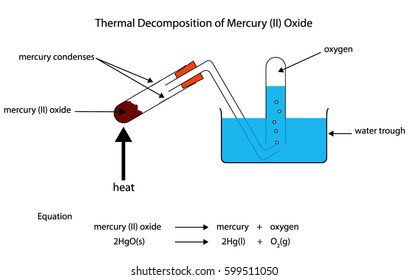 Source: shutterstock.com
Source: shutterstock.com
An example is when baking soda sodium bicarbonate is heated 2N aH CO3s CO2g H 2Og N a2CO3s Here is a video discussion of this reaction. A decomposition reaction has following mechanism. Decomposition is defined as the chemical reaction in which a single compound gives two or more simple substances. Share It On Facebook Twitter Email. Answered Feb 7 2018 by Md samim 952k points selected Feb 7 2018 by sforrest072.
 Source: youtube.com
Source: youtube.com
The process is not reversible. ZnCO3 oversetheatrightarrow. Example 5 Electrolysis of water The breaking down of H 2 O into H 2 and O 2 by passing electricity is known as electrolysis of water. 2N2 O5 4NO2 O2 overal N2 O5 NO2 NO3 fast decomposition NO2 NO3 NONO2 O2 slow NONO3 2NO2 fast Determine rate law and show the mechanism corresponds to first order reaction. Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Give an example each for thermal decomposition and photochemical decomposition reactions. An example of t Continue Reading Related Answer Aman Sharma former Student. The decomposition of potassium chlorate to form potassium chloride and oxygen gas when hated 2KClO 3 sheat2KCl s3O 2 g The decomposition of ferric dioxide into ferric oxide and water on heating. Thermal composition reaction or thermolysis is the decomposition by means of heat. A thermal decomposition reaction occurs when heat is applied to a compound causing it to decompose break down into multiple different chemical substances.
 Source: edurev.in
Source: edurev.in
CuSO4 CuO SO3 H2O SO3 H2SO4. Carbonates decompose into carbon dioxide and an oxide. An example is when baking soda sodium bicarbonate is heated 2N aH CO3s CO2g H 2Og N a2CO3s Here is a video discussion of this reaction. The presence of carbon dioxide gas can be tested using limewater. Ii Thermal decomposition reactions form one of the steps in extraction of metals.
 Source: edurev.in
Source: edurev.in
Thermal Decomposition reaction examples CaCO3 CaO CO2 In the above reaction calcium carbonate when heated for thermal decomposition calcium oxide along with carbon dioxide will produce. Thermal Decomposition Reaction. The digestion of food in the body is an example of decomposition reaction. Examples of thermal dissociation are. Answered Feb 7 2018 by Md samim 952k points selected Feb 7 2018 by sforrest072.
 Source: toppr.com
Source: toppr.com
The equation is represented below. ZnCO3 oversetheatrightarrow. A thermal decomposition reaction occurs when heat is applied to a compound causing it to decompose break down into multiple different chemical substances. These reactions are the endothermic type of reaction as it requires external heat for reaction. Thermal Decomposition Reaction.
 Source: brainly.in
Source: brainly.in
A solid remains in the tube but its identity is now copper oxide CuO. An example is when baking soda sodium bicarbonate is heated 2N aH CO3s CO2g H 2Og N a2CO3s Here is a video discussion of this reaction. Thermal decomposition - such reactions are usually. The decomposition of hydrogen peroxide into oxygen and hydrogen gas is a typical example of the decomposition reaction. Example 5 Electrolysis of water The breaking down of H 2 O into H 2 and O 2 by passing electricity is known as electrolysis of water.
 Source: brainly.in
Source: brainly.in
This process is employed in the manufacturing of quick lime which is an important substance in many industries. The presence of carbon dioxide gas can be tested using limewater. Thermal decomposition reactions are common in everyday life. A common example of a thermal decomposition reaction is provided below. An example of t Continue Reading Related Answer Aman Sharma former Student.
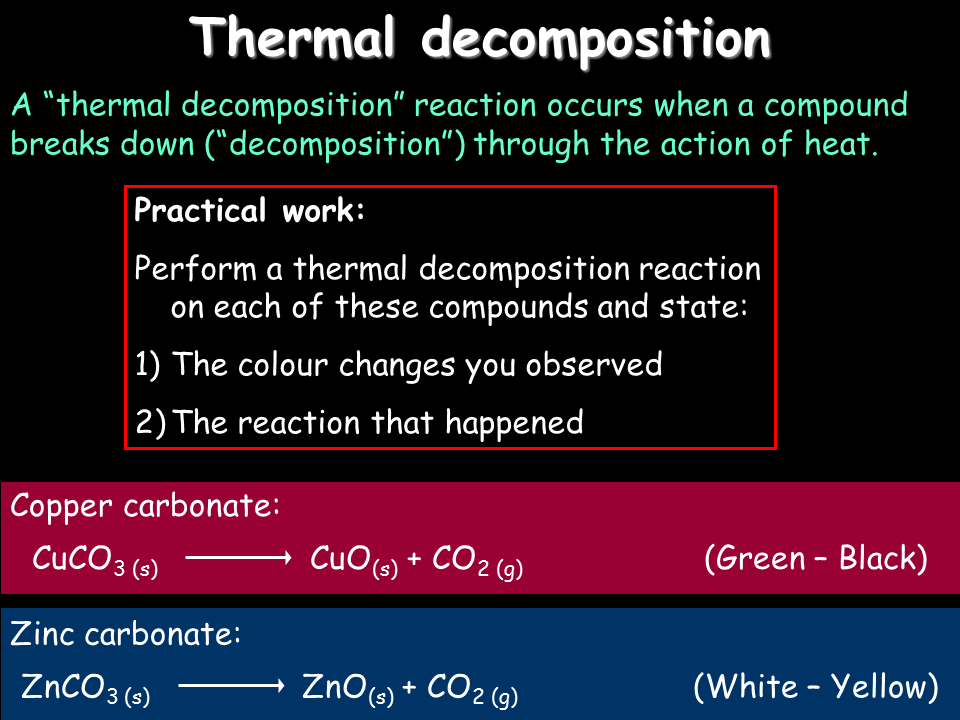
The decomposition of potassium chlorate to form potassium chloride and oxygen gas when hated 2KClO 3 sheat2KCl s3O 2 g The decomposition of ferric dioxide into ferric oxide and water on heating. Chemical reactions and equations. Share It On Facebook Twitter Email. Examples of thermal dissociation are. CaCO 3 CaO CO 2 Combustion.
 Source: brainly.in
Source: brainly.in
Decomposition reaction examples in real life Decomposition reactions are chemical reactions in which a more complex molecule breaks down to make simpler ones. Write relevant balanced chemical equations also. Answered Feb 7 2018 by Md samim 952k points selected Feb 7 2018 by sforrest072. What are the uses of decomposition reactions. A decomposition reaction has following mechanism.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title example of thermal decomposition reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






