Your Gibbs free energy example images are ready. Gibbs free energy example are a topic that is being searched for and liked by netizens today. You can Find and Download the Gibbs free energy example files here. Get all free images.
If you’re looking for gibbs free energy example pictures information connected with to the gibbs free energy example keyword, you have pay a visit to the right site. Our site frequently gives you hints for seeing the maximum quality video and picture content, please kindly hunt and find more informative video articles and images that fit your interests.
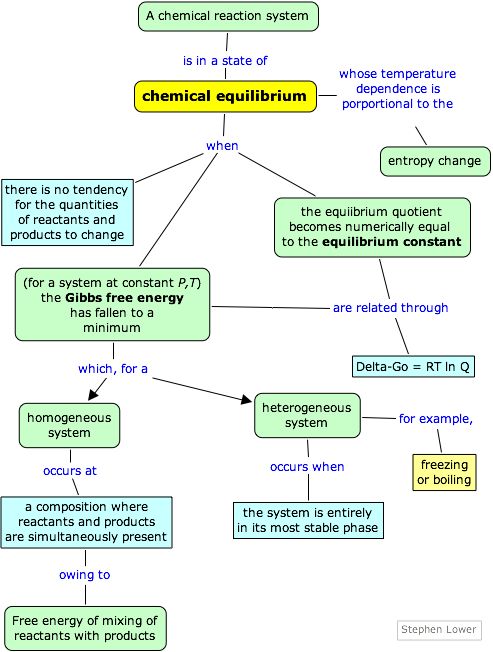
Gibbs Free Energy Example. 1000 Questions Gibbs Free energy. 3042018 There is no disorder Example. This is given by the relationship. Where is enthalpy is temperature in kelvin and is the entropy.
 Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Generator Free Energy Free Energy Projects From pinterest.com
Gibbs Free Energy Equilibrium Constant Enthalpy Entropy Equations Free Energy Generator Free Energy Free Energy Projects From pinterest.com
G H - TSsystem For any spontaneous change G will be negative. DG SdT VdP dN. The maximum work done is the amount of energy produced given by the decrease in the thermodynamic property called Gibbs free energy. Use the results of this calculation to determine the value of Go for this reaction at 25 o C and explain. Give reasons why this compound would be unstable at room temperature. For reaction to be spontaneous Δ G should be negative Δ G 0.
Where is enthalpy is temperature in kelvin and is the entropy.
Entropy and Free Energy are 506b Gibbs Free Energy Example Problem 2 438. 506 Gibbs Free Energy 812. Changing the equations accordingly looks like this. Gibbs free energy or delta G is the enthalpy change of a reaction delta H minus the entropy of the reaction system multiplied by product of temperature t. Gibbs Free Energy Change G Gibbs free energy is a term that combines the effect of enthalpy and entropy into one number The balance between entropy and enthalpy determines the feasibility of a reaction. 501a Enthalpy of Reaction Refresher 232.
 Source: in.pinterest.com
Source: in.pinterest.com
1000 Questions Gibbs Free energy. G H - TSsystem For any spontaneous change G will be negative. It is defined by the Gibbs equation. Δ G Δ H T Δ S. 1000 Questions Gibbs Free energy.
 Source: pinterest.com
Source: pinterest.com
Gibbs free energy has extensive property and a function with a single value. When a process occurs at constant temperature and pressure we can rearrange the second law of thermodynamics and define a new quantity known as Gibbs free energy. 3042018 There is no disorder Example. Entropy and Free Energy are 506b Gibbs Free Energy Example Problem 2 438. G H - TSsystem For any spontaneous change G will be negative.
 Source: pinterest.com
Source: pinterest.com
Gibbs free energy is represented using the symbol and typically has units of. 506 Gibbs Free Energy 812. The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings. This term was used by chemists in the initial years of physical chemistry to portray the forces behind chemical reactions. DG SdT VdP dN.
 Source: pinterest.com
Source: pinterest.com
As another example consider the thermodynamic energy expressed in terms of the Gibbs free energy. Gibbs Free Energy Change G Gibbs free energy is a term that combines the effect of enthalpy and entropy into one number The balance between entropy and enthalpy determines the feasibility of a reaction. You can easily check the consistency of the intensiveextensive variables here. Entropy and Free Energy are 506b Gibbs Free Energy Example Problem 2 438. For reaction to be spontaneous Δ G should be negative Δ G 0.
 Source: pinterest.com
Source: pinterest.com
Gibbs free energy and spontaneity. The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings. This term was used by chemists in the initial years of physical chemistry to portray the forces behind chemical reactions. Also calculates the change in entropy using table of standard entrop. 1 G H T S G 19070 cal 310 K 90 calK G 8830 cal 883 kcal 2.
 Source: pinterest.com
Source: pinterest.com
Maybe one of these molecules bonds with this molecule so you have one of the dark blues. 3042018 There is no disorder Example. 501a Enthalpy of Reaction Refresher 232. Where is enthalpy is temperature in kelvin and is the entropy. You can easily check the consistency of the intensiveextensive variables here.
 Source: pinterest.com
Source: pinterest.com
It is defined by the Gibbs equation. 505 Entropy of the Universe 852. I have to increase the disorder by that amount. Furthermore in 1873 Josiah Willard Gibbs published his paper A Method. Determining if a reaction is spontaneous by calculating the change in Gibbs free energy.
 Source: pinterest.com
Source: pinterest.com
This is given by the relationship. Both Δ H and T Δ S are negative. 510 Standard versus Nonstandard Free Energy Change 1513. DG DH - TDS. The Gibbs function is defined as the enthalpy minus the temperature times the entropy.
 Source: pinterest.com
Source: pinterest.com
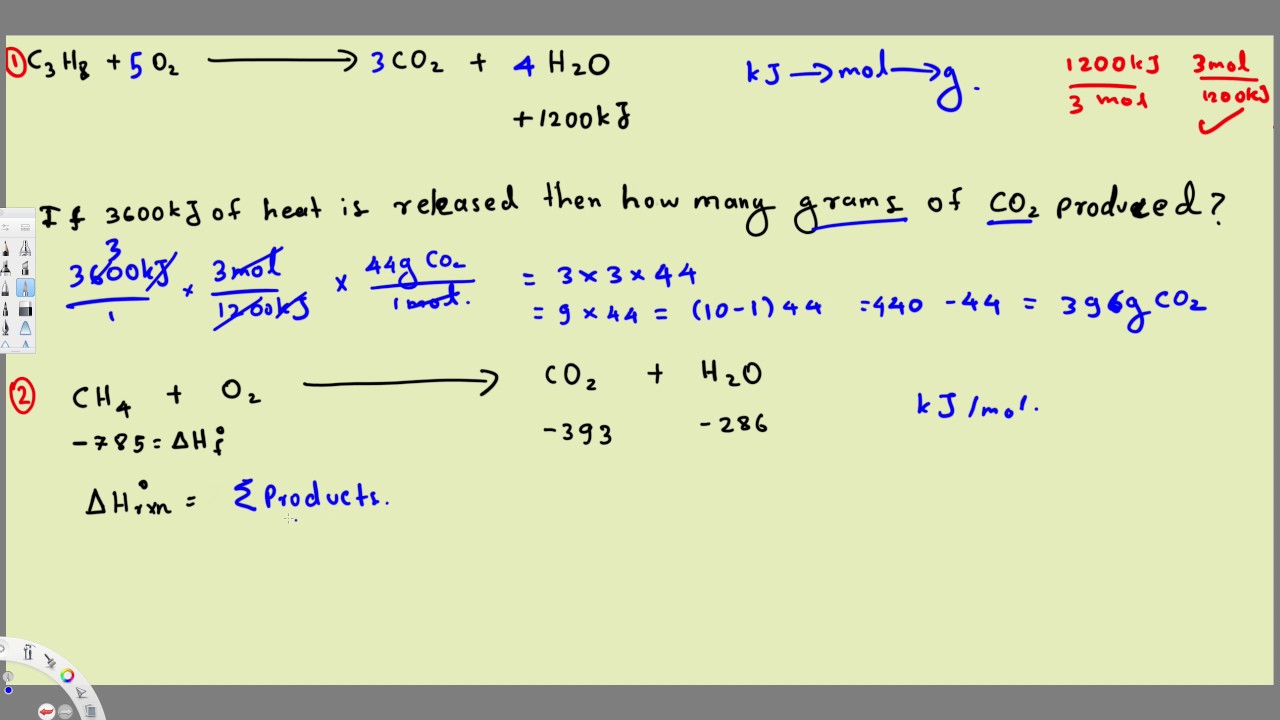
ΔH f of Cl 2 O 7 is 7573 kJ mol -1. Calculate ΔH and ΔS for the dissolution of NH 4 NO 3 in water. G H - TSsystem For any spontaneous change G will be negative. 501a Enthalpy of Reaction Refresher 232. Gibbs Free Energy Practice Problems 5.
 Source: pinterest.com
Source: pinterest.com
The free energy change DG is equal to -TDSuniv and it applies just to a system itself without regard for the surroundings. The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions. The numbers of the examples are the in the HG EX tags on the slides. We shall discuss the energetics of different types of biological structure including small organic molecules membranes nucleic acids and proteins. G H - TSsystem For any spontaneous change G will be negative.
 Source: pinterest.com
Source: pinterest.com
506 Gibbs Free Energy 812. The Gibbs function is defined as the enthalpy minus the temperature times the entropy. 1 G H T S G 19070 cal 310 K 90 calK G 8830 cal 883 kcal 2. History of Gibbs Free Energy. The maximum work done is the amount of energy produced given by the decrease in the thermodynamic property called Gibbs free energy.
 Source: pinterest.com
Source: pinterest.com
This is given by the relationship. 506a Gibbs Free Energy Example Problem 1 512. Determining if a reaction is spontaneous by calculating the change in Gibbs free energy. Calculate the Gibbs free energy change G for the following chemical reaction. Gibbs Free Energy Change G Gibbs free energy is a term that combines the effect of enthalpy and entropy into one number The balance between entropy and enthalpy determines the feasibility of a reaction.
 Source: pinterest.com
Source: pinterest.com
ΔH change in enthalpy. The quantity termed as free energy is an advanced and accurate replacement for the archaic term affinity. Furthermore in 1873 Josiah Willard Gibbs published his paper A Method. As another example consider the thermodynamic energy expressed in terms of the Gibbs free energy. 506 Gibbs Free Energy 812.
 Source: pinterest.com
Source: pinterest.com
G H - TSsystem For any spontaneous change G will be negative. Gibbs free energy or delta G is the enthalpy change of a reaction delta H minus the entropy of the reaction system multiplied by product of temperature t. For reaction to be spontaneous Δ G should be negative Δ G 0. Gibbs free energy is represented using the symbol and typically has units of. Helmholtz and Gibbs Energy Examples These are the examples to be used along with the powerpoint lecture slides.
 Source: pinterest.com
Source: pinterest.com
505 Entropy of the Universe 852. Both Δ H and T Δ S are negative. The quantity termed as free energy is an advanced and accurate replacement for the archaic term affinity. DG SdT VdP dN. Gibbs free energy G Gibbs free energy G standard free energy change ΔG0 and equilibrium constant Keq Problem.
 Source: pinterest.com
Source: pinterest.com
This is given by the relationship. Gibbs free energy G Gibbs free energy G standard free energy change ΔG0 and equilibrium constant Keq Problem. Determining if a reaction is spontaneous by calculating the change in Gibbs free energy. For reaction to be spontaneous Δ G should be negative Δ G 0. DG SdT VdP dN.
 Source: in.pinterest.com
Source: in.pinterest.com
506a Gibbs Free Energy Example Problem 1 512. For a spontaneous process at constant temperature and pressure DG must be negative. Δ G Δ H T Δ S. Both Δ H and T Δ S are negative. A range of examples illustrate when where why and how the Gibbs free energy is such a useful concept.
 Source: pinterest.com
Source: pinterest.com
If theres a change in Gibbs free energy if theres a negative change if theres a downhill direction for Gibbs free energy thats the favored direction for a chemical process or a physical strategy. This is given by the relationship. 1000 Questions Gibbs Free energy. Maybe one of these molecules bonds with this molecule so you have one of the dark blues. When a process occurs at constant temperature and pressure we can rearrange the second law of thermodynamics and define a new quantity known as Gibbs free energy.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title gibbs free energy example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






