Your Le chateliers principle examples images are available in this site. Le chateliers principle examples are a topic that is being searched for and liked by netizens now. You can Get the Le chateliers principle examples files here. Download all royalty-free vectors.
If you’re searching for le chateliers principle examples pictures information linked to the le chateliers principle examples topic, you have pay a visit to the right blog. Our website always provides you with suggestions for refferencing the highest quality video and picture content, please kindly surf and find more informative video content and graphics that match your interests.
Le Chateliers Principle Examples. When a reversible reaction is at equilibrium disturbances in concentration temperature pressure etc will be offset to reach a new equilibrium. Join learners like you already enrolled. A statement of Le Chateliers Principle. Le ChÂteliers Principle Not all situations of equilibrium are alike.
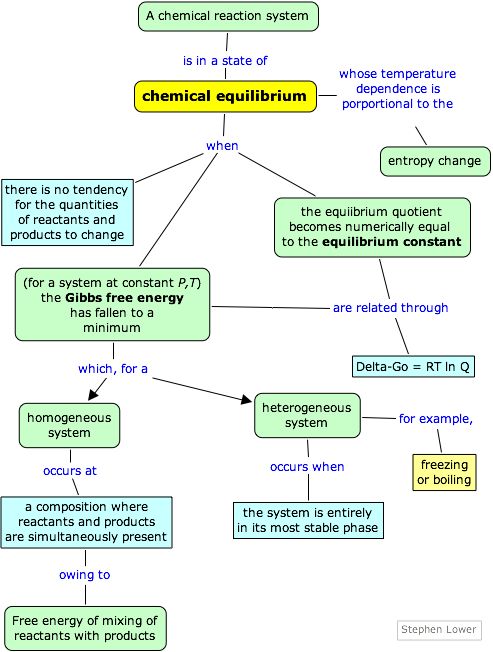 Free Energy And Equilibrium Chemistry Basics Chemistry Lessons Chemistry Classroom From in.pinterest.com
Free Energy And Equilibrium Chemistry Basics Chemistry Lessons Chemistry Classroom From in.pinterest.com
A statement of Le Chateliers Principle. The principle is named after the French chemist Henry Louis Le Chatelier. Depending on certain factors the position of equilibrium may favor one side of the equation or the other. There is an increases the. Le Chateliers Principle In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibria. L ə ʃ æ ˈ t ɛ l j eɪ or US.
Identify how Le Chateliers principle would impact these examples.
It is applied to any chemical reaction that is capable of reaching equilibrium in closed systems. Created by Yuki Jung. It states that If an external stress is applied to a reacting system at equilibrium the system will adjust itself in such a way that the effect of the stress is nullified. Practice using Le Chateliers principle to predict what happens to a reaction when a stress is applied. It is applied to any chemical reaction that is capable of reaching equilibrium in closed systems. A change in one of the variables that describe a system at equilibrium produces a shift in the position of the equilibrium that counteracts the.
 Source: pinterest.com
Source: pinterest.com
See how these chemical systems respond to changes in the concentrations of reactants or products or to changes in temperature. Le Chateliers principles also known as the equilibrium law are used to predict the effect of some changes on a system in chemical equilibrium such as the change in temperature or pressure. The principle is named after the French chemist Henry Louis Le Chatelier. Le Chateliers principle can be stated as follows. It states that If an external stress is applied to a reacting system at equilibrium the system will adjust itself in such a way that the effect of the stress is nullified.
 Source: pinterest.com
Source: pinterest.com
A worked example using Le Chateliers principle to predict how concentrations will shift for different perturbations. Using Le Chateliers Principle with a change of concentration. Choose from many topics skill levels and languages. There is an increases the. Case1 - Exothermic Reaction.
 Source: pinterest.com
Source: pinterest.com
If a company is producing chemicals for sale for example its production managers will attempt to influence reactions in such a way as to favor the forward reaction. Observe several interesting and colorful chemical reactions that are examples of chemical systems at equilibrium. Example includes changing reaction vessel volume changing amount of solid product adding inert gas and adding a catalyst. Join learners like you already enrolled. There is an increases the.
 Source: pinterest.com
Source: pinterest.com
Using Le Chateliers Principle. Choose from many topics skill levels and languages. Le Chateliers Principle In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibria. N 2 g 3H 2 g 2NH 3 g ΔH -92kJ. Le ChÂteliers Principle Not all situations of equilibrium are alike.
 Source: pinterest.com
Source: pinterest.com
Introduction to reaction quotient Qc. How Le Chateliers Principle can be used to predict the effect of disturbances to equilibrium. There is an increases the. A worked example using Le Chateliers principle to predict how concentrations will shift for different perturbations. Le Chateliers principle describes the response of a system in equilibrium to counteract the effects caused by an external agent.
 Source: pinterest.com
Source: pinterest.com
There is an increases the. When a reversible reaction is at equilibrium disturbances in concentration temperature pressure etc will be offset to reach a new equilibrium. The classic example of the practical use of the Le Chatelier principle is the Haber-Bosch process for the synthesis of ammonia in which a balance between low temperature and high pressure must be found. Log K 1 K 2 -ΔHºR 1T 2 - 1T 1 Example. Le ChÂteliers Principle Not all situations of equilibrium are alike.
 Source: pinterest.com
Source: pinterest.com
Le Chateliers principle says that this net reaction will occur in a direction that partially offsets the change. Introduction to reaction quotient Qc. Le Chateliers principle says that this net reaction will occur in a direction that partially offsets the change. Le Chateliers principle - higher tier. The two French chemists Le-Chateliers and Braun in 1884 made a certain generalizations to explain the effect of changes of system in equilibrium due to concentration temperature or pressure.
 Source: pinterest.com
Source: pinterest.com
Le Chateliers Principle on Change of Temperature. Le Chateliers principle can be stated as follows. It follows the forward reaction. Log K 1 K 2 -ΔHºR 1T 2 - 1T 1 Example. Suppose you have an equilibrium established between four substances A B C and D.
 Source: pinterest.com
Source: pinterest.com
Le Chateliers principle pronounced UK. The reaction quotient Q. Le Chateliers Principle is the principle when a stress is applied to a chemical system at equilibrium the equilibrium will shift to relieve the stressIn other words it can be used to predict the direction of a chemical reaction in response to a change in conditions of temperature concentration volume or pressureWhile Le Chateliers principle can be used to. Observe several interesting and colorful chemical reactions that are examples of chemical systems at equilibrium. Le Chateliers Principle Examples of Chemical Equilibria -1- E6 Objective.
 Source: pinterest.com
Source: pinterest.com
In this article we shall study Le-Chateliers principle with examples. Observe several interesting and colorful chemical reactions that are examples of chemical systems at equilibrium. Identify how Le Chateliers principle would impact these examples. Le Chateliers Principle - Practice Problems for Assignment 4 Consider the following equilibrium 1 when answering questions 1 and 2. How Le Chateliers Principle can be used to predict the effect of disturbances to equilibrium.
 Source: pinterest.com
Source: pinterest.com
Introduction to reaction quotient Qc. Introduction to reaction quotient Qc. The equilibrium will shift to the right since there is an increase in the product of gas and since its treated like concentration it goes up. ˈ ʃ ɑː t əl j eɪ also called Chateliers principle or the Equilibrium Law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibriaThe principle is named after French chemist Henry Louis Le Chatelier and sometimes also credited to Karl Ferdinand Braun. Le Chateliers principle says that this net reaction will occur in a direction that partially offsets the change.
 Source: in.pinterest.com
Source: in.pinterest.com
The reaction quotient Q. Example includes changing reaction vessel volume changing amount of solid product adding inert gas and adding a catalyst. In the case of an exothermic reaction a decrease in temperature takes place since it releases energy. Le Chateliers Principle - Practice Problems for Assignment 4 Consider the following equilibrium 1 when answering questions 1 and 2. Le Chateliers Principle In 1884 the French chemist and engineer Henry-Louis Le Chatelier proposed one of the central concepts of chemical equilibria.
 Source: pinterest.com
Source: pinterest.com
A worked example using Le Chateliers principle to predict how concentrations will shift for different perturbations. The generalization is known as Le-Chatelierss principle. Example includes changing reaction vessel volume changing amount of solid product adding inert gas and adding a catalyst. Ad Find the right instructor for you. Case1 - Exothermic Reaction.
 Source: pinterest.com
Source: pinterest.com
How Le Chateliers Principle can be used to predict the effect of disturbances to equilibrium. Discuss Le-Chateliers principle by providing example with suitable chemical reaction and schematic diagram. Introduction to reaction quotient Qc. The classic example of the practical use of the Le Chatelier principle is the Haber-Bosch process for the synthesis of ammonia in which a balance between low temperature and high pressure must be found. Suppose you have an equilibrium established between four substances A B C and D.
 Source: in.pinterest.com
Source: in.pinterest.com
The reaction quotient Q. Example includes changing reaction vessel volume changing amount of solid product adding inert gas and adding a catalyst. Le Chatelier said that equilibrium adjusts the forward and backward. Choose from many topics skill levels and languages. When a reversible reaction is at equilibrium disturbances in concentration temperature pressure etc will be offset to reach a new equilibrium.
 Source: pinterest.com
Source: pinterest.com
A statement of Le Chateliers Principle. In the case of an exothermic reaction a decrease in temperature takes place since it releases energy. ˈ ʃ ɑː t əl j eɪ also called Chateliers principle or the Equilibrium Law is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibriaThe principle is named after French chemist Henry Louis Le Chatelier and sometimes also credited to Karl Ferdinand Braun. Le Chateliers principle can be stated as follows. Le Chateliers principle pronounced UK.
 Source: pinterest.com
Source: pinterest.com
Experts are tested by Chegg as specialists in their subject area. N 2 g 3H 2 g 2NH 3 g ΔH -92kJ. The two French chemists Le-Chateliers and Braun in 1884 made a certain generalizations to explain the effect of changes of system in equilibrium due to concentration temperature or pressure. Using Le Chateliers Principle with a change of concentration. The equilibrium will shift to the right since there is an increase in the product of gas and since its treated like concentration it goes up.
 Source: pinterest.com
Source: pinterest.com
If a reaction at equilibrium is distubred itll shift to minimize disturbance 4. Identify how Le Chateliers principle would impact these examples. Le ChÂteliers Principle Not all situations of equilibrium are alike. Discuss Le-Chateliers principle by providing example with suitable chemical reaction and schematic diagram. The equilibrium will shift to the right since there is an increase in the product of gas and since its treated like concentration it goes up.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title le chateliers principle examples by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






