Your New dea 222 form example images are ready. New dea 222 form example are a topic that is being searched for and liked by netizens now. You can Get the New dea 222 form example files here. Find and Download all royalty-free photos and vectors.
If you’re looking for new dea 222 form example images information related to the new dea 222 form example keyword, you have come to the right site. Our site frequently gives you hints for seeing the highest quality video and image content, please kindly hunt and locate more enlightening video articles and images that fit your interests.
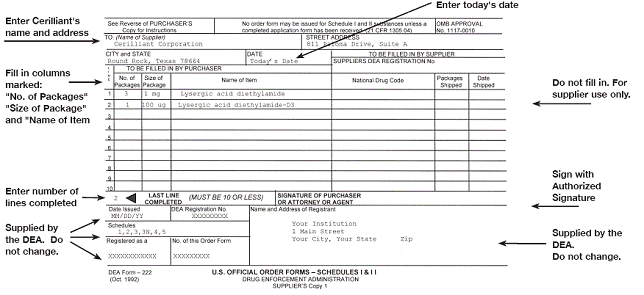
New Dea 222 Form Example. See the example completed form on. If an order cannot be filled in its entirety it may be filled in part and the balance supplied by additional shipments within 60 days following the date of the DEA Form 222. In any case as soon as a registrants supply of triplicate DEA Forms 222 is exhausted the registrant must use the new single-sheet DEA Form 222. Boxes or multiple vial packages - enter 1 for each box or package.

Fillable dea 222 form. Official Order Form for Schedule I and II Controlled Substances DEA Form 222. The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. Title 21 Code of Federal Regulations Part 1305 Subpart BDEA FORM 222 130511 Procedure for obtaining DEA Forms 222. A DEA Forms 222 are issued in mailing envelopes containing either seven or fourteen forms each form containing an original duplicate and triplicate copy respectively Copy 1 Copy 2 and Copy 3. How to order Schedule II and IIN products from Covetrus North America using your 222 DEA Form.
The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section.
Dea form 222 colors. The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. DEA 222 Form Sample New Single-Page John Doe MD. Complete 222 form with supplier name and address. To order Schedule II controlled substances from Midwest Veterinary Supply you must have a current DEA license and a completed Controlled Substance Ordering Form DEA Form 222 on file. The rule provides for a two-year transition period during which the existing triplicate version of the forms may continue to be used.
 Source: pdffiller.com
Source: pdffiller.com
CT DC DE IN IL KY MA MD ME MI NCNH NJ NY. VOID forms with errors and retain. The rule provides for a two-year transition period during which the existing triplicate version of the forms may continue to be used. The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. A Procedure for obtaining DEA Forms 222.
 Source: dvm360.com
Source: dvm360.com
The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. In any case as soon as a registrants supply of triplicate DEA Forms 222 is exhausted the registrant must use the new single-sheet DEA Form 222. Get access to thousands of forms. The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section.
 Source: pdffiller.com
Source: pdffiller.com
If an order cannot be filled in its entirety it may be filled in part and the balance supplied by additional shipments within 60 days following the date of the DEA Form 222. Official Order Form for Schedule I and II Controlled Substances DEA Form 222. This search engine indexes the Drug Enforcement Administration Diversion Control Program Web Site wwwdeadiversionusdojgov only. DEA published a notice of proposed rulemaking about this new format in November 2007 but did not finalize it. Please use SAMPLE DEA FORM - 222 suggested descriptions when submitting your US.
 Source: pdffiller.com
Source: pdffiller.com
The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. When the Drug Enforcement Administration DEA grants the Registrant permission to use Schedule I or II C-I or C-II drugs they will send DEA Form 222 order formsThe Registrant is responsible for securing the Forms 222 and retaining the executed and unexecuted forms. Complete 222 form with supplier name and address. The DEA Form 222 is a triplicate form that is required by the DEA to allow the exchange of controlled substances from the registrant to another. 1 for a box of 10 x 1 ml syringes 3 for 3 boxes of 25 x 1 ml vials etc.

Common 222 Form Errors corresponding to numbers in the sample form below. OFFICIAL ORDER FORM forms to Amatheon. Call our DEA Compliance Team 18882223722. How to order Schedule II and IIN products from Covetrus North America using your 222 DEA Form. Once the DEA Compliance Department gives you the green light to send your 222 form save on postage by using an Amatheon FedEx Pre-Paid label.
 Source: pharmadiversion.com
Source: pharmadiversion.com
In any case as soon as a registrants supply of triplicate DEA Forms 222 is exhausted the registrant must use the new single-sheet DEA Form 222. In any case as soon as a registrants supply of triplicate DEA Forms 222 is exhausted the registrant must use the new single-sheet DEA Form 222. Hit the arrow with the inscription Next to jump from box to box. A Procedure for obtaining DEA Forms 222. When the Drug Enforcement Administration DEA grants the Registrant permission to use Schedule I or II C-I or C-II drugs they will send DEA Form 222 order formsThe Registrant is responsible for securing the Forms 222 and retaining the executed and unexecuted forms.
 Source: youtube.com
Source: youtube.com
DEA published a notice of proposed rulemaking about this new format in November 2007 but did not finalize it. Hit the arrow with the inscription Next to jump from box to box. DEA requires that your 222 form address be the same as the address on your current DEA Certificate. This search engine indexes the Drug Enforcement Administration Diversion Control Program Web Site wwwdeadiversionusdojgov only. Official Order Form for Schedule I and II Controlled Substances DEA Form 222.
 Source: cerilliant.com
Source: cerilliant.com
On February 21 2019 the Drug Enforcement Administration DEA published a Notice of Proposed Rulemaking NPRM New Single-Sheet Format for US. A DEA Forms 222 are issued in mailing envelopes containing either seven or fourteen forms each form containing an original duplicate and triplicate copy respectively Copy 1 Copy 2 and Copy 3. In any case as soon as a registrants supply of triplicate DEA Forms 222 is exhausted the registrant must use the new single-sheet DEA Form 222. The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. The triplicate forms are still good but must be used for the next two years.
 Source: cerilliant.com
Source: cerilliant.com
The rule provides for a two-year transition period during which the existing triplicate version of the forms may continue to be used. Call 1-800-399-VETS for more information. The triplicate forms are still good but must be used for the next two years. Please use SAMPLE DEA FORM - 222 suggested descriptions when submitting your US. Create this form in 5 minutes.

Get access to thousands of forms. Get access to thousands of forms. Pharmaceutical Distribution Center DC in Memphis Tennessee. VIEW SAMPLE OF SINGLE SHEET FORMAT NEW. The rule provides for a two-year transition period during which the existing triplicate version of the forms may continue to be used.

Order your controlled substances and give the original DEA Form-222 to the supplier retain the copy for your own records. Forms cannot have alterations. VIEW SAMPLE OF SINGLE SHEET FORMAT NEW. The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. The DEA Form 222 is a triplicate form that is required by the DEA to allow the exchange of controlled substances from the registrant to another.

The rule provides for a two-year transition period during which the existing triplicate version of the forms may continue to be used. SCHEDULE I Il AMATHEON Animal Health OMB APPROVAL No. The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. OFFICIAL ORDER FORM forms to Amatheon. 1117-0010 See Reverse of Purchasers Copy of Instructions TO.

Please use SAMPLE DEA FORM - 222 suggested descriptions when submitting your US. 1117-0010 See Reverse of Purchasers Copy of Instructions TO. Order your controlled substances and give the original DEA Form-222 to the supplier retain the copy for your own records. Were here to help. Get access to thousands of forms.
 Source: signnow.com
Source: signnow.com
Therefore on October 30 2021 your pharmacy will need to send in the existing triplicate copies of the DEA Form 222 to the Registration Section at the DEA Headquarters and use the new single sheet of a paper DEA Form 222. Avoid having your form returned. DEA published a notice of proposed rulemaking about this new format in November 2007 but did not finalize it. On February 21 2019 the Drug Enforcement Administration DEA published a Notice of Proposed Rulemaking NPRM New Single-Sheet Format for US. CT DC DE IN IL KY MA MD ME MI NCNH NJ NY.
 Source: sasrx.com
Source: sasrx.com
Call our DEA Compliance Team 18882223722. When the Drug Enforcement Administration DEA grants the Registrant permission to use Schedule I or II C-I or C-II drugs they will send DEA Form 222 order formsThe Registrant is responsible for securing the Forms 222 and retaining the executed and unexecuted forms. Official Order Form for Schedule I and II Controlled Substances DEA Form 222. How to order Schedule II and IIN products from Covetrus North America using your 222 DEA Form. The new DEA-222 form will be a single page which will eliminate the need for the triplicate form we are all so used to.
 Source: pdffiller.com
Source: pdffiller.com
1 for a box of 10 x 1 ml syringes 3 for 3 boxes of 25 x 1 ml vials etc. The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. New dea 222 form example. No DEA Form 222 is valid more than 60 days after its execution by the purchaser except as. There is a two-year transition period and in that time the DEA will allow registrants to exhaust their current supply of the old forms.
 Source: pdfsimpli.com
Source: pdfsimpli.com
The Drug Enforcement Administration DEA is amending its regulations to implement a new single-sheet format for DEA Form 222 used by DEA registrants to order schedules I and II controlled substances. The provisions of this part are applicable to the use of triplicate forms except for the specific rules as provided in this section. This search engine indexes the Drug Enforcement Administration Diversion Control Program Web Site wwwdeadiversionusdojgov only. Sample DEA Form is to be used as a reference when filling out the 222 Form. The Drug Enforcement Administration DEA is proposing to amend its regulations to implement a new single-sheet format for order forms DEA Form 222 which are issued by DEA to DEA registrants to allow them to order schedule I andor II controlled substances.
 Source: youtube.com
Source: youtube.com
See the example completed form on. Complete 222 form with supplier name and address. The new DEA-222 form will be a single page which will eliminate the need for the triplicate form we are all so used to. Fill in the necessary boxes which are colored in yellow. VOID forms with errors and retain.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title new dea 222 form example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






