Your Nonpolar covalent bond examples list images are available in this site. Nonpolar covalent bond examples list are a topic that is being searched for and liked by netizens today. You can Get the Nonpolar covalent bond examples list files here. Find and Download all free photos and vectors.
If you’re searching for nonpolar covalent bond examples list images information connected with to the nonpolar covalent bond examples list interest, you have visit the right site. Our site always provides you with suggestions for seeking the maximum quality video and picture content, please kindly hunt and find more enlightening video articles and images that match your interests.
Nonpolar Covalent Bond Examples List. Useful List of Molecules Non-Polar from READE. It occurs whenever the atoms combining have a similar electron affinity. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Over 17 electronegativity difference.
 Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding Types Of Chemical Bonds Youtube From youtube.com
Polar Covalent Bonds And Nonpolar Covalent Bonds Ionic Bonding Types Of Chemical Bonds Youtube From youtube.com
Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Nonpolar Covalent Bond Definition. Nonpolar covalent bonds tend to form between two very similar atoms. Polar covalent bond Hydrogen chloride also called hydrochloric acid is. Ammonium Chloride NH4Cl is a coordinate covalent bond example where both electrons required for bonding are supplied by the same atom. Nitrous oxide nitric oxide ammonia.
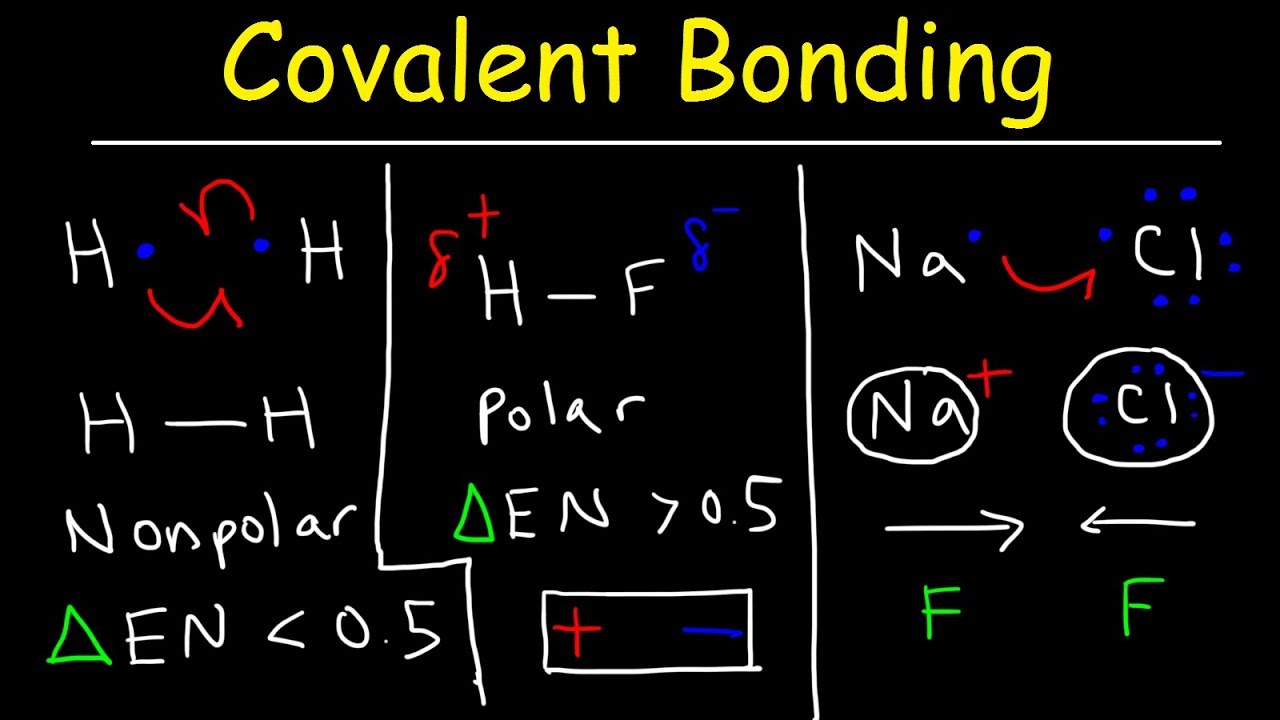
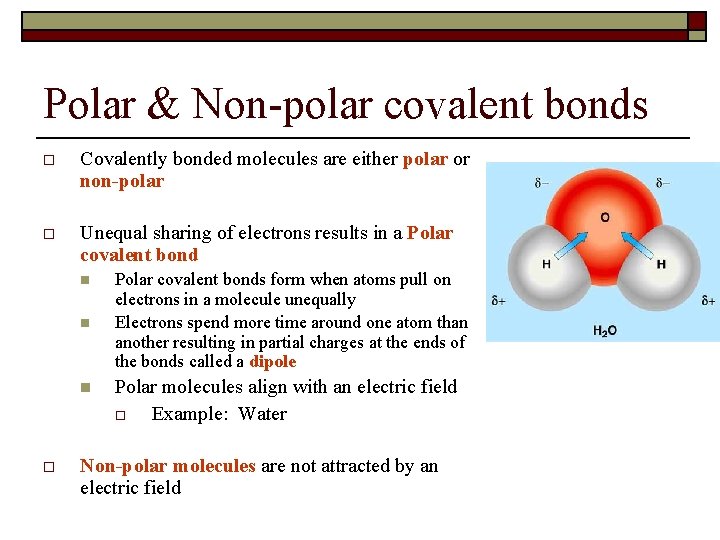
In a nonpolar covalent bond electrons are shared equally between atoms.
When the two atoms share electrons there is also a change of electron density. In polar covalent bond electronegativity the difference between the atoms varies between 04 and 17. Polar Bonds Polar bonds happen when two atoms form a molecule using a covalent bond. Nonpolar covalent bonds are very strong and they require a huge amount of energy to break the bond. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond.
 Source: slidetodoc.com
Source: slidetodoc.com
Nonpolar Covalent Bonds for example can be found in gas molecules such as hydrogen gas nitrogen gas and so on. This type of covalent bond is formed when two atoms share an equal number of electrons. In a molecule when electronegativity difference is less than 04 are referred as nonpolar covalent bonds. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na and negative charged Cl- ions. Polar Bonds Polar bonds happen when two atoms form a molecule using a covalent bond.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
In polar covalent bonds unequal attraction of electrons is seen. Nitrous oxide nitric oxide ammonia. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. If the electrons are not shared equally then there will be a. In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar.
 Source: slideplayer.com
Source: slideplayer.com
Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. A notable exception is carbon monoxide CO. Over 17 electronegativity difference. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ammonium Chloride NH4Cl is a coordinate covalent bond example where both electrons required for bonding are supplied by the same atom.
 Source: bio.libretexts.org
Source: bio.libretexts.org
A notable exception is carbon monoxide CO. Nonpolar covalent bonds are very strong and they require a huge amount of energy to break the bond. Nonpolar Covalent Bonds for example can be found in gas molecules such as hydrogen gas nitrogen gas and so on. The bonds between hydrogen and oxygen in water are an example of this type of bond. Polar Bonds Polar bonds happen when two atoms form a molecule using a covalent bond.
 Source: quora.com
Source: quora.com
Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. σ single covalent bond shared pair is between the tow atoms s orbital with s orbital. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. Nonpolar Covalent Bonds for example can be found in gas molecules such as hydrogen gas nitrogen gas and so on.
 Source: chemistrylearner.com
Source: chemistrylearner.com
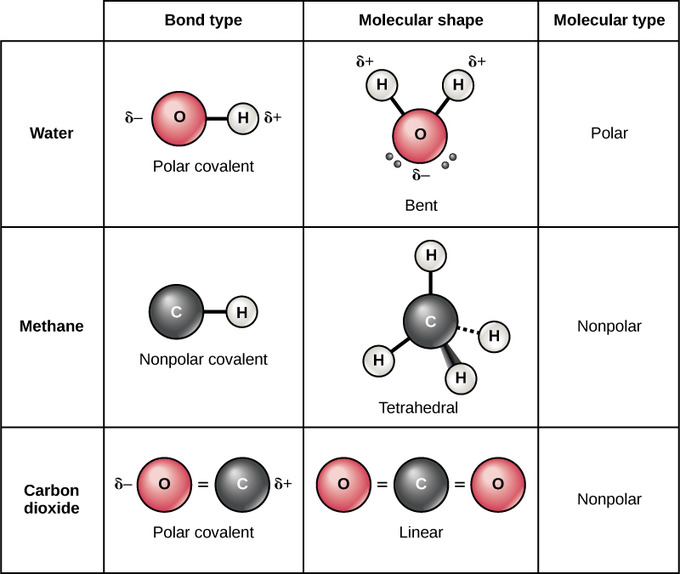
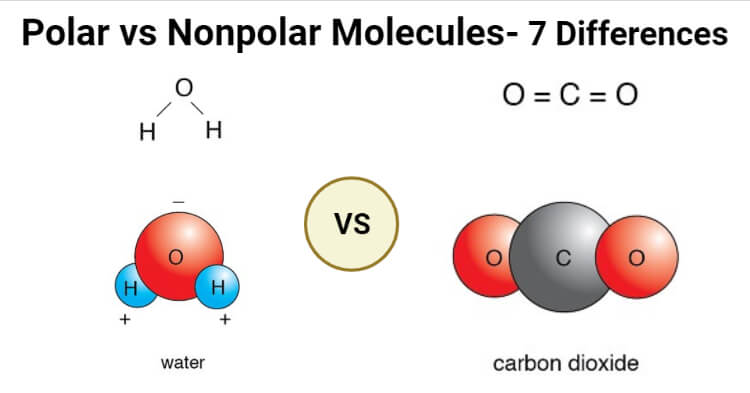
Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline. Electrons are shared differently in ionic and covalent bonds. The charges all cancel out each other. Polar and Nonpolar Covalent Bonds. CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Covalent bonds can be non-polar or polar and react to electrostatic charges. In H-H each H atom has an electronegativity value of 21 therefore the covalent bond between them is considered nonpolar. Useful List of Molecules Non-Polar from READE. Non-polar covalent bonds appear between two atoms of the same element or between different elements that equally share. Polar and Nonpolar Covalent Bonds.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Nonpolar covalent bond electronegativity scale. For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline. In a nonpolar covalent bond electrons are shared equally between atoms. Nonpolar Covalent Bond Definition.
 Source: slideplayer.com
Source: slideplayer.com
For example two hydrogen atoms bond covalently to form an H 2 molecule but each hydrogen atom in the H2 molecule has two electrons stabilizing it giving each atom the same number of valence electrons as the noble gas He. Nonpolar covalent bonds are very strong bonds requiring a large. Nonpolar Covalent - 14 images - figure 3 4 formation of a covalent bond fluorine f has covalent bond examples several examples of covalent polar covalent bond definition and examples is scl4f2 polar or nonpolar. Answer 1 of 2. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms.
 Source: study.com
Source: study.com
The charges all cancel out each other. The bonds between hydrogen and oxygen in water are an example of this type of bond. Most carbon compounds are nonpolar. Useful List of Molecules Non-Polar from READE. Here is a table listing molecules with polar and non.
 Source: youtube.com
Source: youtube.com
Nonpolar covalent bonds are very strong bonds requiring a large. CO_2 O_2 CH_3 DNA non-polar amino acids Covalent bonds are common in the molecules of living organisms. σ single covalent bond shared pair is between the tow atoms s orbital with s orbital. Covalent bonds can be non-polar or polar and react to electrostatic charges. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Electrons are shared differently in ionic and covalent bonds. Nonpolar covalent41-17 electronegativity difference. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. In a molecule when electronegativity difference is less than 04 are referred as nonpolar covalent bonds. The more electrons they share the stronger the bond will be.
 Source: slideplayer.com
Source: slideplayer.com
If the difference in electronegativity between two atoms is 04 or less the bond formed between the two atoms is a nonpolar covalent bond. Most carbon compounds are nonpolar. Nonpolar Covalent - 14 images - figure 3 4 formation of a covalent bond fluorine f has covalent bond examples several examples of covalent polar covalent bond definition and examples is scl4f2 polar or nonpolar. Examples of homonuclear nonpolar molecules are oxygen O 2 nitrogen N 2 and ozone O 3. In polar covalent bonds unequal attraction of electrons is seen.
 Source: study.com
Source: study.com
Bonding between chlorine and bromine. Useful List of Molecules Non-Polar from READE. Carbon monoxide is a linear molecule but the. Nonpolar Covalent Bond Definition. Types of Covalent Bonds.
 Source: users.stlcc.edu
Source: users.stlcc.edu
The bonds between hydrogen and oxygen in water are an example of this type of bond. In polar covalent bonds unequal attraction of electrons is seen. Polar covalent bond Hydrogen chloride also called hydrochloric acid is. Polar and Nonpolar Covalent Bonds. Nonpolar covalent bonds tend to form between two very similar atoms.
 Source: thechemistrynotes.com
Source: thechemistrynotes.com
Nonpolar covalent bonds are very powerful bonds demanding a large amount of energy to break. Nonpolar covalent bonds are very strong bonds requiring a large. Other nonpolar molecules include carbon dioxide CO 2 and the organic molecules methane CH 4 toluene and gasoline. Nonpolar covalent bond electronegativity scale. The charges all cancel out each other.
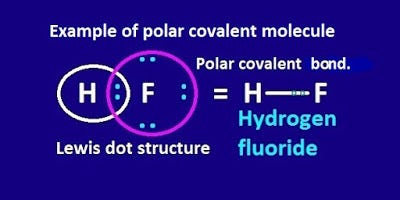 Source: kgghosh1990.medium.com
Source: kgghosh1990.medium.com
The difference in electronegativity between two atoms is zero. When the two atoms share electrons there is also a change of electron density. Polarization of Covalent Bonds It is observed that in the sigma bonds between two different atoms the electron cloud is always closer to the more electronegative of the two atoms participating in the sigma bond. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds. A covalent bond is a form of chemical bond that requires the exchange of electron pairs between atoms.
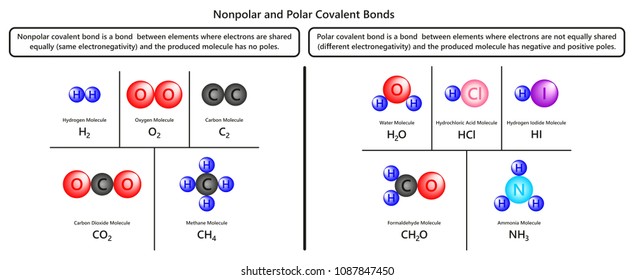 Source: shutterstock.com
Source: shutterstock.com
Nonpolar covalent bonds are very powerful bonds demanding a large amount of energy to break. If the difference in electronegativity between two atoms is 04 or less the bond formed between the two atoms is a nonpolar covalent bond. In polar covalent bonds unequal attraction of electrons is seen. Nonpolar covalent41-17 electronegativity difference. In more simple words a nonpolar covalent bond is formed when atoms share an equal number of electrons between them.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nonpolar covalent bond examples list by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






