Your Oxidation half reaction example images are available in this site. Oxidation half reaction example are a topic that is being searched for and liked by netizens today. You can Get the Oxidation half reaction example files here. Find and Download all free vectors.
If you’re searching for oxidation half reaction example images information related to the oxidation half reaction example keyword, you have visit the ideal site. Our website frequently gives you suggestions for viewing the highest quality video and image content, please kindly hunt and locate more enlightening video content and graphics that fit your interests.
Oxidation Half Reaction Example. The two half-reactions are then multiplied by suitable integers so that the total number of electrons gained in the reduction half-reaction is equal to the total number of electrons lost in the oxidation half-reaction. Cu 2 2e Cu. 2Zn aq Cus Zns Cu2aq reduction half-reaction-Zn2 aq. Fe 2 is oxidized to Fe 3 by hydrogen peroxide when an acid is present.
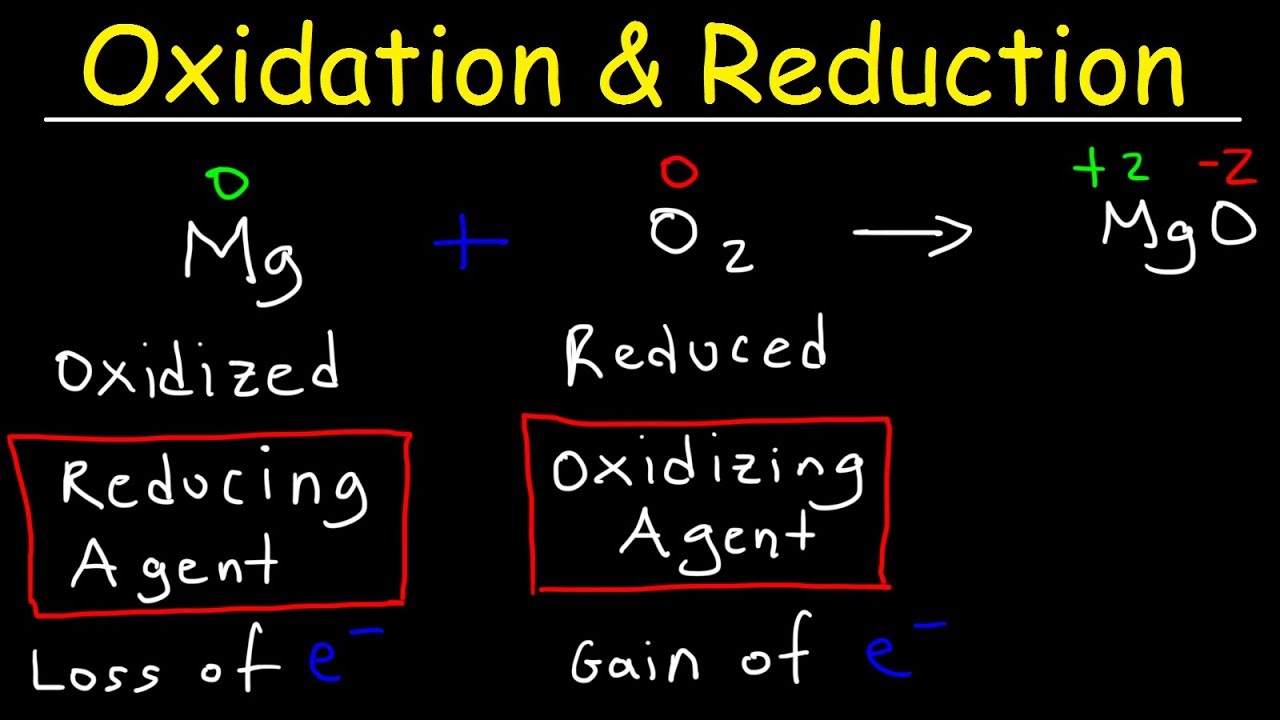 Oxidation And Reduction Redox Reactions And Electrochemistry Chemistry Khan Academy Youtube Chemistry Basics College Chemistry Chemistry From pinterest.com
Oxidation And Reduction Redox Reactions And Electrochemistry Chemistry Khan Academy Youtube Chemistry Basics College Chemistry Chemistry From pinterest.com
If your oxidation number is reducing you are being reduced. Here are a number of highest rated Oxidation Reduction Examples pictures on internet. This reaction is provided below. Thus copper is displaced from the copper sulfate solution by zinc in a redox reaction. CH 3 CH 2 OH CH 3 CO 2 H 4 e-. Notice how half reactions are always listed as reductions that is as gaining electrons.
It can be seen from this example that an oxidation reaction is one in which a species loses electrons and a reduction reaction is one in which a species gains electrons.
We allow this nice of Oxidation Reduction Examples graphic could possibly be the most trending topic as soon as we allowance it in google plus. Which one is being reduced. In this case multiply all coefficients in the oxidation half-reaction by three. So oxidation reactions need not involve oxygen. 34HNO 3 Cu 2 O m 2CuNO 3 2 H 2 O 2H 2e 12HNO 3 3Cu 2 O m 6CuNO 3 2 3H 2 O 6H 6e To make both half-reactions show the same number of electrons multiply all the coefficients in the reduction half-reaction by two. Zn Zn 2 2e The reduction half-reaction can be written as.
 Source: pinterest.com
Source: pinterest.com
Aluminum is oxidized to Al 2 O 3 in this reaction which means that Fe 2 O 3 must be the oxidizing agent. So now lets set up both of the half reactions the oxidized half reaction for aluminum and then the reduction for hydrogen. Oxidation Example For example in this reaction CuOMg MgOCu electrons are gained by copper and lost by magnesium. Oxidation Reduction Examples. Pyruvate CO2 2H 2 e malate.
 Source: in.pinterest.com
Source: in.pinterest.com
Oxidation-Reduction or redox reactions occur when elements in a chemical reaction gain or lose electrons causing an increase or decrease in oxidation numbers. Als is being oxidized. The silver is being reduced its oxidation number going from 1 to zero. Reduction reactions always occur in conjunction with oxidation reactions hence the name redox reactions. Thus to make hydrofluoric gas hydrogen and fluorine are required.
 Source: pinterest.com
Source: pinterest.com
In the following redox reaction which species is being oxidized. Answer 1 of 6. An Oxidation half reaction is when a substance loses or donates electrons to an oxidizing agent that in turn accepts electrons and is reduced in a reduction half reaction. 34HNO 3 Cu 2 O m 2CuNO 3 2 H 2 O 2H 2e 12HNO 3 3Cu 2 O m 6CuNO 3 2 3H 2 O 6H 6e To make both half-reactions show the same number of electrons multiply all the coefficients in the reduction half-reaction by two. Examples of this category of oxidation Electrochemistry studies oxidation-reduction reactions.
 Source: in.pinterest.com
Source: in.pinterest.com
Oxidation Example For example in this reaction CuOMg MgOCu electrons are gained by copper and lost by magnesium. NAD 2H 2 e NADH H. The coppers oxidation number went from zero to 2 so it was oxidized in the reaction. Reaction between Iron and Hydrogen Peroxide. Oxidation Reduction Examples.
 Source: pinterest.com
Source: pinterest.com
Half Reaction Method Examples Example 1. If your oxidation number is reducing you are being reduced. Its submitted by executive in the best field. Agaq is being reduced. Another example could be that of hydrofluoric acid where fluorine is reduced and hydrogen is oxidized H 2 F 2 2HF.
 Source: pinterest.com
Source: pinterest.com
Just as metal atoms get oxidized by losing electrons the biomolecules. Pyruvate CO2 2H 2 e malate. The two half-reactions are then multiplied by suitable integers so that the total number of electrons gained in the reduction half-reaction is equal to the total number of electrons lost in the oxidation half-reaction. In this second half reaction the element hydrogen H 2 has been formed. 2HNO 3 3H 3em NO 2H 2 O 2HNO 3.
 Source: in.pinterest.com
Source: in.pinterest.com
Cu 2 2e Cu. The silver is being reduced its oxidation number going from 1 to zero. To do that identify the atoms which get reduced and get oxidized. 34HNO 3 Cu 2 O m 2CuNO 3 2 H 2 O 2H 2e 12HNO 3 3Cu 2 O m 6CuNO 3 2 3H 2 O 6H 6e To make both half-reactions show the same number of electrons multiply all the coefficients in the reduction half-reaction by two. Answer 1 of 6.
 Source: pinterest.com
Source: pinterest.com
Thus copper is displaced from the copper sulfate solution by zinc in a redox reaction. If your oxidation number is reducing you are being reduced. Addition of electronegative element. Thus copper is displaced from the copper sulfate solution by zinc in a redox reaction. We identified it from honorable source.
 Source: pinterest.com
Source: pinterest.com
The coppers oxidation number went from zero to 2 so it was oxidized in the reaction. Zn Zn 2 2e The reduction half-reaction can be written as. By assigning oxidation numbers we can pick out the oxidation and reduction halves of the reaction. This lesson walks through how to write half reactions for oxidation and reduction given a particular redox reaction. Addition of electronegative element.
 Source: pinterest.com
Source: pinterest.com
It can be seen from this example that an oxidation reaction is one in which a species loses electrons and a reduction reaction is one in which a species gains electrons. So first for the aluminum we have an aluminum solid and its half reaction. Als is being oxidized. Fe S FeS oxidation of Iron 3. Addition of electronegative element.
 Source: in.pinterest.com
Source: in.pinterest.com
2Zn aq Cus Zns Cu2aq reduction half-reaction-Zn2 aq. Its submitted by executive in the best field. We allow this nice of Oxidation Reduction Examples graphic could possibly be the most trending topic as soon as we allowance it in google plus. Thus copper is displaced from the copper sulfate solution by zinc in a redox reaction. If necessary assign oxidation numbers and then write two half-reactions oxidation and reduction The example equation is in acidic conditions.
 Source: pinterest.com
Source: pinterest.com
H 2 F 2 2 HF In this reaction hydrogen is being oxidized and fluorine is being reduced. As electrons lost by one species must be accepted by another then both. Conversely Fe 2 O 3 is reduced to iron metal which means that aluminum must be the reducing agent. H 2 2 H 2 e - F 2 2 e - 2 F -. 34HNO 3 Cu 2 O m 2CuNO 3 2 H 2 O 2H 2e 12HNO 3 3Cu 2 O m 6CuNO 3 2 3H 2 O 6H 6e To make both half-reactions show the same number of electrons multiply all the coefficients in the reduction half-reaction by two.
 Source: in.pinterest.com
Source: in.pinterest.com
Half Reaction Method Examples Example 1. 2HNO 3 3H 3em NO 2H 2 O 2HNO 3. Als is being oxidized. Thus to make hydrofluoric gas hydrogen and fluorine are required. The two half-reactions are then multiplied by suitable integers so that the total number of electrons gained in the reduction half-reaction is equal to the total number of electrons lost in the oxidation half-reaction.
 Source: in.pinterest.com
Source: in.pinterest.com
One of the basic reasons that the concept of oxidation-reduction reactions helps to correlate chemical knowledge is that a particular oxidation or reduction can often be carried out by a wide variety of oxidizing or reducing agents. Conversely Fe 2 O 3 is reduced to iron metal which means that aluminum must be the reducing agent. If necessary assign oxidation numbers and then write two half-reactions oxidation and reduction The example equation is in acidic conditions. One of the basic reasons that the concept of oxidation-reduction reactions helps to correlate chemical knowledge is that a particular oxidation or reduction can often be carried out by a wide variety of oxidizing or reducing agents. The oxidation half-reaction therefore formally corresponds to the loss of four electrons by one of the carbon atoms.
 Source: pinterest.com
Source: pinterest.com
H 2 F 2 2 HF In this reaction hydrogen is being oxidized and fluorine is being reduced. The reaction may be better understood if it is written in terms of two half-reactions. Reduction of the ironIII ion to the ironII ion by four different reducing. 2Zn aq Cus Zns Cu2aq reduction half-reaction-Zn2 aq. The Half Equation Method is used to balance these reactionsRedox reactions usually occur in.
 Source: pinterest.com
Source: pinterest.com
Als is being oxidized. By assigning oxidation numbers we can pick out the oxidation and reduction halves of the reaction. Another example could be that of hydrofluoric acid where fluorine is reduced and hydrogen is oxidized H 2 F 2 2HF. So oxidation reactions need not involve oxygen. Oxidation-Reduction or redox reactions occur when elements in a chemical reaction gain or lose electrons causing an increase or decrease in oxidation numbers.
 Source: pinterest.com
Source: pinterest.com
Zn Zn 2 2e The reduction half-reaction can be written as. Its submitted by executive in the best field. To do that identify the atoms which get reduced and get oxidized. Reduction of the ironIII ion to the ironII ion by four different reducing. The reaction that occurs between carbon dioxide and water to produce carbohydrates is an example of an oxidation reaction.
 Source: pinterest.com
Source: pinterest.com
Thus to make hydrofluoric gas hydrogen and fluorine are required. Classical Idea of Oxidation and Reduction reactions. 2Zn aq Cus Zns Cu2aq reduction half-reaction-Zn2 aq. To do that identify the atoms which get reduced and get oxidized. We identified it from honorable source.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title oxidation half reaction example by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






